Part 8: Pediatric Advanced Life Support
Abstract
The American Heart Association and the American Academy of Pediatrics provide these pediatric advanced life support guidelines focusing on resuscitation during cardiopulmonary resuscitation and emergency cardiovascular care. These guidelines are intended to be a resource for health care professionals to identify and treat infants and children up to 18 years of age (excluding newborn infants) in the prearrest, intra-arrest, and post–cardiac arrest states as well as select other emergency care situations. These guidelines apply to infants and children in various settings, including the community, prehospital environments, and hospital environments. Topics presented include ventilation and advanced airway strategies during cardiopulmonary resuscitation; drug administration and weight-based dosing of medications during cardiopulmonary resuscitation; energy doses for defibrillation; measuring cardiopulmonary resuscitation physiology and quality; extracorporeal cardiopulmonary resuscitation; post–cardiac arrest care related to management of core temperature, blood pressure, oxygenation/ventilation, neurologic monitoring, and seizures; neurological prognostication post–cardiac arrest; post–cardiac arrest survivorship; family presence during cardiopulmonary resuscitation; evaluation of sudden unexplained cardiac arrest; management of shock types; airway/intubation management; arrhythmia management including bradycardia and tachycardia (narrow and wide complex); treatment of myocarditis/cardiomyopathies; resuscitation of patients with single ventricle congenital heart disease; management of pulmonary hypertension; and management of traumatic cardiac arrest. Lastly, important gaps in resuscitation science knowledge are identified, aiming to encourage further scientific inquiry and provide additional evidence for future pediatric advanced life support guidelines.
Top 10 Take-Home Messages
- High-quality cardiopulmonary resuscitation (CPR) is the foundation of pediatric advanced life support (PALS) resuscitation for health care professionals. We reaffirm the key components of high-quality CPR: providing adequate chest compression rate and depth, minimizing interruptions in CPR, allowing full chest recoil between compressions, and providing sufficient ventilation for the pediatric patient population while avoiding excessive ventilation.
- For initial nonshockable rhythms, administering epinephrine as soon as possible is associated with favorable outcomes for infants and children in cardiac arrest.
- Rapid defibrillation remains the priority for cardiac arrest with initial shockable rhythms. Administer epinephrine if defibrillation is not immediately possible.
- For infants and children with continuous invasive arterial blood pressure monitoring in place during CPR, diastolic blood pressure targets of ≥25 mm Hg in infants and ≥30 mm Hg in children at least 1 year of age are now included as hemodynamic goals of high-quality cardiopulmonary resuscitation.
- End-tidal carbon dioxide (ETCO2) can be an indicator of CPR quality, although the use of specific ETCO2 cutoff values to guide termination of resuscitation in infants and children is not advised.
- Preventing hyperthermia is a critical component of post–cardiac arrest care. Avoiding central temperatures greater than 37.5 °C can improve neurological outcomes in infants and children who remain comatose following cardiac arrest.
- For infants and children, new data support maintaining post–cardiac arrest systolic and mean arterial blood pressure greater than the 10th percentile for age and sex.
- Neuroprognostication after cardiac arrest in infants and children requires multiple modalities be assessed at various timepoints throughout the post–cardiac arrest period. Single tests conducted in isolation carry a risk of inaccurately predicting neurologic outcomes.
- After discharge from the hospital, cardiac arrest survivors often have ongoing physical, cognitive, and behavioral challenges and require evaluation for appropriate therapies and interventions.
- New data support the use of IV sotalol as an anti-arrhythmic to treat infants and children with supraventricular tachycardia (SVT) and cardiopulmonary compromise that is unresponsive to vagal maneuvers, IV adenosine, and electrical synchronized cardioversion when expert consultation is not available.
Preamble
Every year, more than 7000 out-of-hospital cardiac arrests (OHCA) and approximately 20,000 in-hospital cardiac arrests (IHCA) occur in infants and children across the United States.1-3 In the United States, approximately 80% of emergency medical services-treated OHCA occur in the home.4 In contrast, the majority of IHCA arrests in infants and children occur in the intensive care unit (ICU) environment with incidence rates ranging from 1.8% for general pediatric ICU admissions (2011–2013)5 to 3.1% in pediatric cardiac ICUs, although variability exists between centers.6 Understanding the full spectrum of neurologic outcomes during the weeks to months after cardiac arrest across the pediatric age spectrum is limited by variability in reporting metrics and time to follow-up across studies. Survival to hospital discharge rates for OHCA range from 6% to 38%4,7,8 . Although data on favorable neurologic outcomes among survivors of OHCA are limited, the most recent Cardiac Arrest Registry to Enhance Survival data demonstrate rates of 5.7% in infants to 12.8% in older children.4 In contrast, survival to hospital discharge for IHCA ranges from 38% for pulseless cardiac arrest to 66% for nonpulseless cardiac arrest.6,9 Definitions of neurologic outcome vary across studies, and neurologic outcomes are defined by different measures (eg, Vineland Adaptive Behavioral Score or Pediatric Cerebral Performance Category [PCPC]) and different thresholds. While guideline recommendations are based on review of combined data, readers should pay attention to individual study measures when looking for detailed information. Grossly favorable neurologic outcomes in survivors of IHCA based on the PCPC scoring system are much higher than OHCA neurological outcome rates at approximately 64% to 89%.5,10
These guidelines contain recommendations for PALS, excluding newborn infants, and are based on the best available resuscitation science. The Chain of Survival requires coordinated efforts from medical professionals in various disciplines, as well as from lay responders, emergency dispatchers, and first responders in the case of OHCA. Pediatric guidance and recommendations are provided in “Part 6: Pediatric Basic Life Support.”11 Recommendations for resuscitation training are in “Part 12: Resuscitation Education Science.”12 Recommendations about systems of care are in “Part 4: Systems of Care.”13 Recommendations for special circumstances are in “Part 10: Special Circumstances.”14 Considerations around ethics are provided in “Part 3: Ethics.”15
Scope of the Guidelines
These guidelines are intended to be a resource for health care professionals to identify and treat infants and children in the prearrest, intra-arrest, and post–cardiac arrest states. These apply to infants and children in multiple settings: the community, prehospital, and hospital environments. Prearrest, intra-arrest, and post–cardiac arrest topics are reviewed, including cardiac arrest in special circumstances, such as infants and children with congenital heart disease.
For the purposes of the PALS guidelines, pediatric patients are infants and children up to 18 years of age. In contrast, pediatric basic life support guidelines apply to infants and children without signs of puberty. Neither pediatric advanced nor basic life support guidelines address the resuscitation of newborn infants, who are transitioning from a fluid-filled to an air-filled environment. Resuscitation of the newborn infant is addressed in “Part 5: Neonatal Resuscitation”16. Although pediatric basic and advanced life support guidelines may be applied to newborn infants younger than 28 days of age based on pathophysiology and institutional practice, neonatal guidelines should be followed at birth to address unique aspects of transitional physiology.17
Organization of the Pediatric Writing Committee
The PALS Writing Group consisted of pediatric clinicians from the American Heart Association (AHA) and the American Academy of Pediatrics (AAP) including intensivists, cardiac intensivists, cardiologists, emergency medicine physicians, and nurses. A call for candidates was distributed to the AHA Emergency Cardiovascular Care (ECC) Committee and AAP subject matter experts, and volunteers with recognized expertise in pediatric resuscitation were nominated by the writing group co-chairs. Writing group members were selected by the AHA ECC Science Subcommittee and AAP Executive Committee and then approved by the AHA Manuscript Oversight Committee. The AHA and AAP have rigorous conflict of interest policies and procedures to minimize the risk of bias or improper influence during the development of the guidelines. Before their appointment, writing group members and peer reviewers disclosed all commercial relationships and other potential (including intellectual) conflicts. Writing group members whose research informed guideline recommendations were required to declare those conflicts during discussions and abstain from voting on those specific recommendations. This process is described more fully in “Part 2: Evidence Evaluation and Guidelines Development.”18 Comprehensive disclosure information for writing group members and peer reviewers is listed in Appendixes 1(link opens in new window) and 2(link opens in new window).
Methodology and Evidence Review
These pediatric guidelines are based on the extensive evidence evaluation performed in conjunction with the International Liaison Committee on Resuscitation (ILCOR) and affiliated ILCOR member councils. Three different types of evidence reviews (systematic reviews, scoping reviews, and evidence updates) were used in the 2025 process. This process is described more fully in “Part 2: Evidence Evaluation and Guidelines Development.”18
Class of Recommendation and Level of Evidence
The writing group reviewed all relevant and current AHA guidelines for CPR and ECC and all relevant ILCOR consensus on CPR and ECC science with treatment recommendations from 2020, 2022, 2023, and 2024.19-22 Evidence and recommendations were reviewed to determine if current guidelines should be reaffirmed, revised, or retired or if new recommendations were needed. The writing group then drafted, reviewed, and approved recommendations, assigning a class of recommendation (COR; ie, strength) and level of evidence (LOE; ie, quality, certainty) to each. Criteria for each COR and LOE are described in Table 1.
Open table in a new window.
Guideline Structure
The 2025 Guidelines are organized in discrete modules of information on specific topics or management issues.23 Each modular knowledge chunk includes a table of recommendations using standard AHA nomenclature of COR and LOE. Recommendations are presented in order of COR: most potential benefit (Class 1), followed by lesser certainty of benefit (Class 2), and finally no benefit or potential for harm (Class 3). Following the COR, recommendations are ordered by the certainty of supporting LOE: Level A (high-quality randomized controlled trials) to Level C-EO (expert opinion). This order does not reflect the order in which care should be provided.
A brief synopsis is provided to put the recommendations into context with important background information and overarching management or treatment concepts. Recommendation-specific supportive text clarifies the rationale and key study data supporting the recommendations. When appropriate, illustrations are included. Hyperlinked references are provided to facilitate quick access and review.
Document Review and Approval
The writing group consists of AHA and AAP representatives who voted on and approved all guideline recommendations. The guideline was submitted for blinded peer review to 10 subject matter experts nominated by the AHA and AAP. Before their appointment, all peer reviewers were required to disclose relationships with industry and any other conflicts of interest, and all disclosures were reviewed by AHA staff. The guideline was also reviewed and approved for publication by the AHA Science Advisory and Coordinating Committee, the AHA Executive Committee, and the AAP Board of Directors. Comprehensive disclosure information for peer reviewers is listed in Appendixes 1(link opens in new window) and 2(link opens in new window).
These recommendations supersede the last full set of AHA recommendations for PALS24 made in 2020 unless otherwise specified. For topics that did not undergo full evidence review by the 2025 writing group, recommendations, recommendation supportive text and references from the 2020 pediatric basic and advanced life support guidelines24 were not updated and were carried over and remain as the current guidelines for 2025. These topics are noted within the synopsis of their respective sections.
Major Concepts
The epidemiology, pathophysiology, and common etiologies of pediatric cardiac arrest are distinct from adult and neonatal cardiac arrest. Cardiac arrest in infants and children does not usually result from a primary cardiac cause; rather, it is most frequently the result of progressive respiratory failure or shock. In these patients, a variable period of physiologic deterioration ultimately leads to cardiac arrest. Among children with congenital and acquired heart disease, cardiac arrest is more often related to underlying cardiac disease although the etiology is rarely related to coronary insufficiency or ischemia as is commonly observed in the adult population.
Outcomes for pediatric IHCA improved dramatically between 2000 and 2009.1 Subsequent reports of temporal changes in rates of survival to hospital discharge have been variable with marginal improvements.2 Data from the Get With the Guidelines-Resuscitation Registry, a large multicenter, hospital-based cardiac arrest registry, show that survival to hospital discharge from pulseless pediatric cardiac arrest increased from 19% in 2000 and 44% in 2022.3 Survival increased on average 0.67% per year and then plateaued in 2010.4 New directions of research and therapy may be required to improve cardiac arrest survival. In addition, more cardiac arrest events now occur in an ICU setting, which suggests that patients at risk for cardiac arrest are being identified sooner and transferred to a higher level of care before cardiac arrest.2,4
Survival rates from OHCA remain less encouraging. In a recent analysis of the Cardiac Arrest Registry to Enhance Survival, a multicenter OHCA registry, annual survival to hospital discharge of pediatric OHCA through 2022 ranged from 6.6% for infants younger than 1 year of age up to 17.3% for children 13 to 18 years of age.5 Survival rates have increased since earlier data from the Resuscitation Outcomes Consortium Epidemiological Registry demonstrated survival rates from 3.3% for infants less than 1 year of age to 8.9% for children 13 to 18 years of age.6 In this registry, survival from OHCA was higher in regions with more arrests that were witnessed by emergency medical services and with higher lay responder CPR rates, stressing the importance of early recognition and treatment of these patients.6
As survival rates from pediatric cardiac arrest have increased, there has been a shift in focus to neurodevelopmental, physical, and emotional outcomes of survivors. Studies demonstrate that a quarter of patients with favorable outcomes have global cognitive impairment and that 85% of older children who were reported to have favorable outcomes have selective neuropsychological deficits at 1-year follow-up.7
The Cardiac Arrest Chain of Survival
Historically, cardiac arrest care has largely focused on the management of the cardiac arrest itself, highlighting high-quality CPR, early defibrillation, and effective teamwork. However, there are aspects of prearrest and post–cardiac arrest care that are critical to improve outcomes. As pediatric IHCA survival rates have plateaued2, the prevention of cardiac arrest becomes even more important. IHCA prevention includes early recognition and treatment of cardiac arrest such as children undergoing high-risk procedures (eg, infants undergoing cardiac surgery or catheterization procedures), children with high-risk diagnoses (eg, shock, pulmonary hypertension, or acute respiratory distress syndrome), and children with severely abnormal vital signs or other signs of deterioration. In the out-of-hospital environment, lay responder CPR training, sudden unexpected infant death prevention, safety initiatives (eg, bike helmet laws), and early access to emergency care are imperative. When OHCA occurs, early lay responder CPR is critical in improving outcomes.
Following resuscitation from cardiac arrest, management of the post–cardiac arrest syndrome (which may include brain dysfunction, myocardial dysfunction with low cardiac output, and ischemia/reperfusion injury) is important to avoid known contributors to secondary injury, such as hypotension.8,9 There is recognition of growing differential use of the terms return of spontaneous circulation (ROSC) versus return of circulation (ROC) in the literature. For the purposes of these guidelines, after CPR, when ROC is achieved by native cardiac function, we use ROSC; when it has been achieved by either mechanical support (eg, extracorporeal membrane oxygenation, ECMO) or native cardiac function, we use ROC. Accurate neuroprognostication is important to guide caregiver discussions and decision-making.10 Finally, given the high risk of neurodevelopmental impairment in cardiac arrest survivors, early referral for rehabilitation assessment and intervention is key.
A single Cardiac Arrest Chain of Survival (Figure 1) that supports the paradigm of prevention and early recognition through recovery after cardiac arrest has now been standardized across infants, children, and adults (outside of neonatal care).
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In infants and children with out-of-hospital cardiac arrest, it is reasonable to perform bag-mask ventilation rather than advanced airway interventions (tracheal intubation or supraglottic airway [SGA] placement). |
| 2b | C-LD | 2. In infants and children with in-hospital cardiac arrest who do not have an advanced airway in place, it may be reasonable to perform bag-mask ventilation or advanced airway interventions (tracheal intubation or SGA placement). |
| 2b | C-LD | 3. When performing CPR in infants and children with an advanced airway in place, it may be reasonable to target a ventilation rate range of 20–30 breaths/minute (1 breath every 2–3 seconds), accounting for age and clinical characteristics. Hyperventilation may compromise hemodynamics. |
Synopsis
Most pediatric IHCA and OHCA are precipitated by respiratory deterioration or shock. Thus, airway management and effective ventilation are fundamental components of pediatric resuscitation. Although most patients can be successfully ventilated with bag-mask ventilation, this method requires interruptions in chest compressions and is associated with risk of aspiration and barotrauma. Advanced airway interventions, such as tracheal intubation or SGA placement, may improve ventilation, reduce the risk of aspiration, and enable uninterrupted compression delivery. However, airway placement may interrupt the delivery of compressions or result in a malpositioned device, with catastrophic consequences if unrecognized. Advanced airway placement requires specialized equipment and skilled health care professionals and may be difficult for professionals who do not routinely intubate children.
In children receiving CPR with an advanced airway in place, provision of adequate minute ventilation while avoiding hyperventilation and associated deleterious hemodynamic effects is critical. There are limited data regarding ventilation rates during pediatric CPR and determination of optimal ventilation rates deserves further study. Other components of intra-arrest ventilation, such as the appropriate peak end-expiratory pressure or tidal volume, have not been studied extensively.
Recommendation-Specific Supportive Text
- A clinical trial showed that tracheal intubation and bag-mask ventilation achieve similar rates of survival with good neurologic function in pediatric patients with OHCA.1 Three propensity-matched retrospective studies show similar rates of survival to discharge and survival with good neurologic function when comparing advanced airway placement (tracheal intubation or SGA) with bag-mask ventilation in pediatric OHCA.2-4 A fourth propensity-matched study demonstrated lower 1-month survival and survival with favorable neurologic status with advanced airway placement (tracheal intubation or SGA) when compared to bag-mask ventilation.5 No differences have been observed in outcomes between SGA and tracheal intubation in pediatric OHCA.2,3,6
- There are limited data to compare outcomes between bag-mask ventilation versus tracheal intubation in the management of IHCA, and there are no studies of pediatric SGA use during in-hospital CPR. A propensity-matched retrospective cohort study found that intubation during cardiac arrest was associated with decreased survival to hospital discharge compared to no intubation.7 Though there may be specific circumstances or populations in which early advanced airway interventions are beneficial (eg, children for whom bag-mask ventilation is technically difficult, children with severe lung disease), data supporting alternative approaches to these situations are lacking.
- One small, multicenter observational study of intubated pediatric patients found that ventilation rates (at least 30 breaths/min in children younger than 1 year of age, at least 25 breaths/min in older children) were associated with improved rates of ROSC and survival to discharge.8 A ventilation rate of 20 to 30 breaths per minute was selected to account for (1) higher physiologic respiratory rates in children, (2) the preponderance of respiratory etiologies in pediatric arrest, (3) improved associations with survival associations, and (4) to avoid higher ventilation rates that were associated with lower systolic blood pressures during CPR.8
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Rapid initiation of vascular access (IV or IO) is recommended for drug administration in infants and children with cardiac arrest. |
| 1 | C-EO | 2. Health care professionals caring for infants and children with cardiac arrest should choose the initial type of vascular access route (IV or IO) based on availability, expertise, and timeliness. |
Synopsis
Administration of vasoactive and antiarrhythmic medications is a key component of PALS during CPR. Medications, such as epinephrine, have historically been administered via multiple routes, including endotracheal, intravenous (IV) and intraosseous (IO). Due to limited transalveolar drug absorption and limited pulmonary blood flow during CPR, IV and IO administration of medications are preferred over the endotracheal route.1,2 Selecting the timeliest route to deliver medication based on clinical needs and available resources is appropriate.
Recommendation-Specific Supportive Text
- Timely provision of vasoactive and antiarrhythmic medications during cardiac arrest is dependent on rapid securement of vascular access across all pediatric age ranges. IV or IO administration of epinephrine is preferred over endotracheal administration when possible.1,2
- IO access is a rapid, safe, and an effective initial vascular access route for pediatric cardiac arrest.3-5 A recent systematic review did not identify any studies comparing IV versus IO for vascular access in pediatric cardiac arrest.6 Two retrospective nontraumatic OHCA registry studies showed that younger, more severely ill patients with lower rates of survival were more likely to have received IO access while older patients with higher rates of survival were more likely to have received IV access.7,8 Health care professionals should consider resource availability, expertise, and timeliness (time to confirmed vascular access) when choosing vascular access routes for drug administration.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. For infants and children in cardiac arrest with initial nonshockable rhythm, it is reasonable to administer the initial dose of epinephrine as early as possible. |
| 2b | C-LD | 2.For infants and children in cardiac arrest with initial shockable rhythm, it may be reasonable to administer epinephrine after 2 attempts at defibrillation or sooner, only when rapid defibrillation is not possible. |
| 2b | C-LD | 3. For infants and children in cardiac arrest in any setting, it may be reasonable to administer epinephrine every 3–5 minutes until ROSC is achieved. |
| 2b | C-LD | 4. For infants and children with shock-refractory VF/pVT, either amiodarone or lidocaine may be considered. |
| 3: No Benefit | B-NR | 5. Routine administration of sodium bicarbonate is not recommended for infants and children in cardiac arrest, except in special circumstances (eg, sodium channel blocker toxicity or hyperkalemia). |
| 3: No Benefit | B-NR | 6. Routine administration of calcium is not recommended for infants and children in cardiac arrest, except for special circumstances (eg, hypocalcemia, calcium channel blocker overdose, or hyperkalemia). |
Synopsis
Administration of medications is a key component of PALS. Vasoactive agents, such as epinephrine, are used to increase coronary perfusion, primarily through alpha-1 receptor agonism. The optimal timing of initial dosing may differ in shockable cardiac arrest given the need to prioritize defibrillation. Antiarrhythmic agents are used to treat cardiac arrest with ventricular fibrillation (VF) and pulseless ventricular tachycardia (pVT). The existing literature does not support routine use of other adjunctive therapies such as sodium bicarbonate and calcium except in special circumstances.
Recommendation-Specific Supportive Text
- A recent meta-analysis of retrospective observational studies showed that shorter time to administration of epinephrine during pediatric cardiac arrest with initial nonshockable rhythm was associated with favorable outcomes.9-14
- The optimal timing of administration of epinephrine in relation to defibrillation in pediatric cardiac arrest with shockable rhythm is unknown9 and may differ from nonshockable arrest given the need to prioritize defibrillation. Observational studies of pediatric OHCA have included patients with shockable rhythm, but none reported the relationship between initial epinephrine and timing of defibrillation.11,13,15,16 Two observational studies in adults with shockable IHCA showed that early epinephrine, defined as before first defibrillation or within 2 minutes after first defibrillation, respectively, was associated with worse outcomes.17,18 No studies have examined the effect of epinephrine for pediatric shockable arrest when early defibrillation is not possible.
- The optimal interval between doses of epinephrine for pediatric cardiac arrest is unknown.9 Observational studies for conventional CPR have shown mixed results. One study demonstrated that average intervals greater than 5 minutes between doses were associated with increased survival compared to 1 to 5 minute intervals.19 A second study showed that average epinephrine intervals of 3 to less than 5 minutes were associated with increased survival.20 Two additional studies found that intervals of less than 3 minutes between epinephrine doses were associated with better outcomes.21,22 For pediatric patients undergoing extracorporeal cardiopulmonary resuscitation (ECPR), one observational study showed that after the first 10 minutes of CPR, survivors received fewer epinephrine doses during each subsequent 10-minute interval compared to nonsurvivors. There was no difference in survival between epinephrine dosing intervals of ≤5 minutes and greater than 5 minutes during minutes 10 to 30 of CPR on adjusted analysis.23
- In an observational study of pediatric IHCA with VF/pVT, administration of lidocaine was associated with higher rates of ROSC and 24-hour survival although neither lidocaine nor amiodarone significantly affected the odds of survival to hospital discharge.24 A subsequent propensity-matched study of pediatric IHCA with initial VF/pVT demonstrated no difference in outcomes between patients who received lidocaine compared to amiodarone.25
- A meta-analysis of sodium bicarbonate administration during pediatric IHCA demonstrated lower rates of survival to hospital discharge in patients who received bicarbonate.26-33 A subsequent propensity score-weighted cohort study found that sodium bicarbonate was associated with a lower rate of hospital survival, but no difference in rates of ROSC were observed.34 However, these studies do not account for the fact that patients with longer durations of resuscitation, which are associated with lower survival rates, have greater exposure to medications like sodium bicarbonate. This phenomenon, known as resuscitation time bias, may falsely implicate the medication with poor survival if timing of drug administration is not accounted for in the analysis.35 There are special circumstances in which bicarbonate is used, such as sodium channel blocker toxicity (eg, tricyclic antidepressants and cocaine)36 and hyperkalemia. While a comprehensive list of special circumstances in which sodium bicarbonate may be beneficial has not been determined, it is important to note that sodium bicarbonate is routinely used in some of these circumstances (eg, hyperkalemia) which may significantly limit the emergence of new evidence due to lack of equipoise.37
- A recent meta-analysis examining the administration of calcium during pediatric IHCA demonstrated decreased survival to hospital discharge with calcium administration.32,38-40 Two subsequent studies in pediatric IHCA and arrests managed in the emergency department showed similar associations.41,42 However, as noted for sodium bicarbonate, none of the studies accounted for resuscitation time bias and calcium may be falsely implicated in the decreased survival rates in patients who undergo prolonged resuscitation.35 There are special circumstances in which calcium administration is used, such as hypocalcemia, calcium channel blocker overdose, and hyperkalemia. It is important to note that calcium is routinely used in some of these circumstances (eg, hyperkalemia) which may significantly limit the emergence of new evidence due to lack of equipoise.37
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. For infants and children in cardiac arrest with initial nonshockable rhythm, it is reasonable to administer the initial dose of epinephrine as early as possible. |
| 2b | C-LD | 2.For infants and children in cardiac arrest with initial shockable rhythm, it may be reasonable to administer epinephrine after 2 attempts at defibrillation or sooner, only when rapid defibrillation is not possible. |
| 2b | C-LD | 3. For infants and children in cardiac arrest in any setting, it may be reasonable to administer epinephrine every 3–5 minutes until ROSC is achieved. |
Synopsis
Medication dosing for children is based on weight, which is often difficult to obtain in an emergency setting. There are numerous approaches to estimating weight when an actual weight cannot be obtained.43
This topic was last reviewed in the 2020 AHA Guidelines for CPR and ECC. These recommendations have not been re-reviewed and updated for this edition of the Guidelines.44
Recommendation-Specific Supportive Text
- There are many theoretical concerns about the use of actual body weight (especially in overweight or obese patients).45-47 However, there are no data about the safety and efficacy of adjusting medication dosing in obese patients. Such adjustments could result in inaccurate dosing of medications.48,49
- Several studies suggest that inclusion of body habitus or anthropometric measurements further refines and improves weight estimations using length-based measures.43 However, there are considerable variations in these methods, and the training required to employ these measures may not be practical in every context.
- Cognitive aids such as the Broselow, PAWPER XL, and Mercy tapes can assist in the accurate approximation of body weight (described as being within 10% to 20% of measured total body weight). Several studies demonstrated high variability of weight estimates, with a tendency toward underestimation of total body weight yet closely approximating ideal body weight.47,50,51
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In infants and children experiencing cardiac arrest with VF/pVT, it is reasonable to use an initial dose of 2–4 J/kg of monophasic or biphasic energy for defibrillation; for ease of teaching, it is reasonable to use an initial dose of 2 J/kg. |
| 2b | C-LD | 2. In infants and children experiencing cardiac arrest with refractory VF/pVT, it may be reasonable to attempt subsequent defibrillation doses of 4 J/kg of monophasic or biphasic energy, and higher levels may be considered, though not to exceed 10 J/kg or the adult maximum dose. |
| 2b | C-LD | 3. In infants and children experiencing cardiac arrest with refractory VF/pVT, a single defibrillation dose of monophasic or biphasic energy may be considered over sequential (stacked) shocks, followed by resumption of chest compressions. |
Synopsis
The proportion of cardiac arrests with initial VF/pVT steadily increases throughout childhood into adolescence yet remains lower than adults.1,2 In infants and children, cardiac arrests due to an initial rhythm of VF/pVT have better outcomes than cardiac arrests due to initial nonshockable rhythms.3 In addition, development of VF/pVT after the initiation of CPR for an initial nonshockable rhythm (subsequent VF/pVT) is associated with worse outcomes compared to patients in whom VF/pVT is the initial pulseless rhythm.1 Timely defibrillation is the definitive treatment for VF/pVT and the shorter the duration of VF/pVT before defibrillation, the more likely the shock will result in a perfusing rhythm.3 Biphasic defibrillators require lower energy to terminate VF/pVT and have fewer side effects than monophasic defibrillators.4,5 Stacked shocks (the delivery of a rapid series of sequential defibrillations for VF/pVT via one set of pads) are associated with higher rates of VF/pVT termination in some adult studies; however, this has not been well studied in pediatrics.6-9
Recommendation-Specific Supportive Text
- A systematic review10 found insufficient data to determine a relationship between optimal initial energy dose and outcomes. An IHCA case series11 concluded that 2 J/kg was effective in terminating the majority of VF, but neither subsequent rhythm nor outcome was reported. An observational study of IHCA12 showed a higher initial energy dose of ≥3 to 5 J/kg was less effective than 1 to 3 J/kg in achieving ROSC. One observational IHCA study13 did not identify a specific initial energy dose associated with successful VF/pVT termination, while another observational IHCA study14 found that 2 J/kg was an ineffective initial dose compared to higher dose ranges of 2.5 to 3 J/kg, especially for secondary VF. A registry study of pediatric IHCA with initial VF/pVT evaluated children who received energy doses less than 1.7 J/kg, 1.7 to 2.5 J/kg or greater than 2.5 J/kg. They found that children ≤12 years of age who received initial energy doses of less than 1.7 J/kg or greater than 2.5 J/kg had lower rates of survival to hospital discharge compared to those who received 1.7 to 2.5 J/kg. Furthermore, all children ≤18 years of age who received initial energy doses of greater than 2.5 J/kg for initial VF had lower survival rates compared to those who received 1.7 to 2.5 J/kg.15
- Two IHCA studies13,14 suggest that defibrillation doses greater than 2 J/kg may be needed for subsequent shocks to terminate VF. Human and animal studies demonstrate higher defibrillation doses up to 10 J/kg can be provided without significant harm to the myocardium.14,16-21 Maximum energy doses as recommended for adults (see “Part 9: Advanced Life Support” recommendations on defibrillation22) may vary depending on monophasic versus biphasic defibrillators as well as specific manufacturer recommendations.23
- There are limited data for the use of sequential (stacked) shocks, where a series of rapid sequential defibrillations is provided without resumption of compressions between shocks, for pediatric VF/pVT.6,24-26 An IHCA study evaluating transthoracic impedance during defibrillation of children ≥8 years of age suggests that stacked shocks do not improve defibrillation success.6 Notably, stacked shocks lead to longer periods where chest compressions are not being performed.
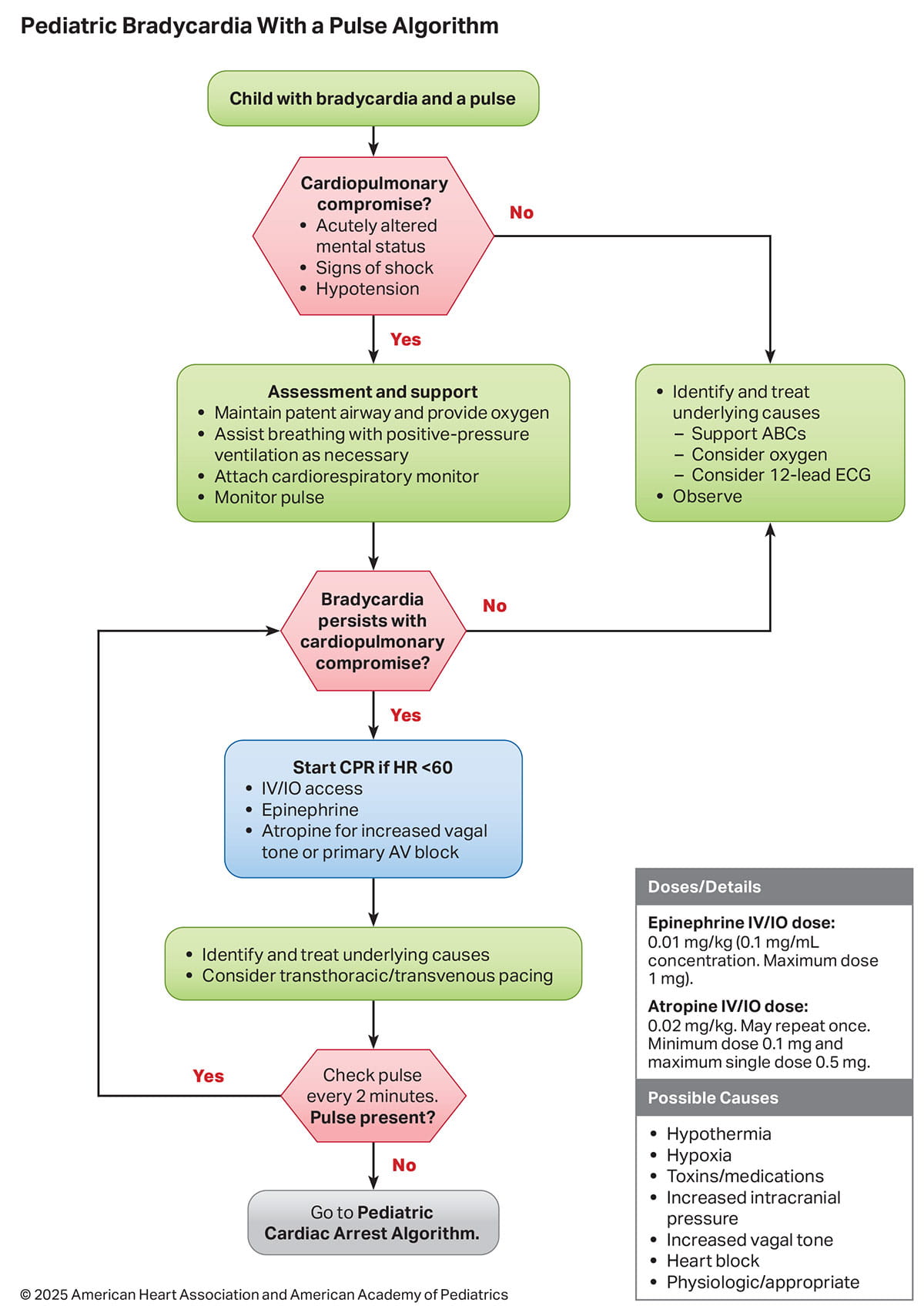
Figure 2 shows the Pediatric Cardiac Arrest Algorithm.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. For infants and children with continuous invasive arterial blood pressure monitoring in place during CPR, it is reasonable for health care professionals to use diastolic blood pressure to assess the child’s response to resuscitation efforts. |
| 2b | B-NR | 2. For infants and children with invasive airways in place during CPR, ETCO2 monitoring may be considered to monitor CPR quality. |
| 2b | C-LD | 3. For infants and children with continuous invasive arterial blood pressure monitoring in place during CPR, it may be reasonable for health care professionals to target a diastolic blood pressure of ≥25 mm Hg in infants and ≥30 mm Hg in children ≥1 year of age. |
| 2b | C-LD | 4. The usefulness of noninvasive cerebral oxygenation monitoring via near-infrared spectroscopy during CPR in infants and children is not well established. |
| 2b | C-EO | 5. It may be reasonable for the rescuer to use CPR feedback devices in infants and children to optimize adequate chest compression rate and depth as part of a continuous resuscitation quality improvement system. |
| 2b | C-EO | 6. When appropriately trained personnel are available, echocardiography may be considered to identify potentially treatable causes of cardiac arrest in infants and children, such as cardiac tamponade and inadequate ventricular filling, but the potential benefits should be weighed against the known deleterious consequences of interrupting chest compressions. |
| 3: Harm | C-LD | 7. A specific ETCO2 cutoff value alone should not be used as an indication to end resuscitative efforts in infants and children. |
Synopsis
Initiating and maintaining high-quality CPR are associated with improved rates of ROSC, survival to hospital discharge, and favorable neurologic outcome, yet measured CPR quality is often suboptimal.1-3 Noninvasive and invasive monitoring techniques may be used to assess CPR quality and the patient’s physiologic response to resuscitation. Invasive arterial blood pressure monitoring during CPR reveals compression and medication-generated pressures.4,5 ETCO2 reflects both the cardiac output produced and ventilation efficacy and may provide feedback on the quality of CPR.6 A sudden rise in ETCO2 may be an early sign of ROSC.7 CPR feedback devices (ie, audio, and audiovisual devices) may improve compression rate, depth, and recoil within a system of training and quality assurance for high-quality CPR. Point-of-care ultrasound during CPR, specifically echocardiography, has been considered for the identification of reversible causes of arrest. Technologies that are under evaluation to assess resuscitation quality include noninvasive measures of cerebral oxygenation, such as near-infrared spectroscopy, which measures regional oxygen saturation and does not require pulsatile flow.
Recommendation-Specific Supportive Text
1 and 3. A multicenter observational study of children with IHCA demonstrated superior outcomes with higher diastolic blood pressure (≥25 mm Hg in infants less than 1 year of age and ≥30 mm Hg in older children ≥1 year of age) during the first 10 minutes of CPR.4 In a prospective multicenter validation study in 413 children, these thresholds were associated with higher relative risk of ROSC (adjusted relative risk 1.49; 1.13–1.97) and survival to hospital discharge ((adjusted relative risk 1.32; 1.01–1.74).8,9 A secondary analysis of the same trial observed that patients who had an increase of ≥5 mm Hg in diastolic blood pressure in response to the first dose of epinephrine had higher rates of ROSC.5 Clinical data regarding the efficacy or appropriate means of prospectively targeting diastolic blood pressure during CPR are lacking.
2. Higher ETCO2 values during CPR are associated with ROSC.10,11 A previous multicenter study of 43 children with IHCA did not identify an association between mean ETCO2 and survival outcomes.12 However, a recent prospective multicenter study in 234 children evaluated average ETCO2 during the first 10 minutes of in-hospital CPR.9,13 On multivariable analysis, ETCO2 ≥20 mm Hg was associated with higher odds of ROSC and survival to discharge and higher intra-arrest blood pressures, but not CPR quality metrics. A single-center study found that higher median event ETCO2 was associated with markers of high-quality CPR—chest compression rate less than 140 compressions per minute (P less than 0.0001) and chest compression fractions of 90% to 100% (P less than 0.0001).11
4. One small single-center study of cerebral near-infrared spectroscopy (n=23) monitored during pediatric cardiac arrest demonstrated that higher median regional cerebral oxygen saturation during the overall CPR event and in the last 5 minutes of CPR was associated with higher rates of ROSC, but not survival to discharge.14 A multicenter study of 92 pediatric patients at 3 sites demonstrated an association between a higher median event regional cerebral oxygen saturation and ROSC, survival to hospital discharge, and survival with favorable neurologic outcome.15
5. A simulation trial of pediatric health care professionals demonstrated a significant improvement in chest compression depth and rate compliance when they received visual feedback (compared to no feedback), although overall compression quality remained poor16 One small observational study of 8 children with IHCA did not find an association between CPR with or without audiovisual feedback and survival to discharge, although feedback decreased excessive compression rates.17
6. Several case series have evaluated the use of bedside echocardiography during pediatric cardiac arrest to identify reversible causes of cardiac arrest and potentially direct management. However, data are very limited and the utility of echocardiography to direct care during pediatric CPR is unknown. Potential risks of echocardiography during CPR include prolonged interruptions in chest compressions.18-20
7. A single-center study found that 42% of infants and children with a median event ETCO2 less than 20 mm Hg achieved ROSC.11 A prospective, multicenter study of ETCO2 during the first 10 minutes of CPR found that 47.5% of infants and children with ETCO2 less than 20 mm Hg and 47.6% of those with ETCO2 less than 10 mm Hg survived to hospital discharge.13 Given these variable associations between low ETCO2 values during CPR and survival outcomes, there is potential harm in making termination of resuscitation decisions based on ETCO2 alone.13,21
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. ECPR may be considered for infants and children with IHCA refractory to conventional CPR in selected populations in clinical settings with ECPR protocols and expertise. |
Synopsis
ECPR is the rapid deployment of venoarterial ECMO for refractory cardiac arrest. This has been defined as the initiation of ECMO flow during active CPR or within 20 minutes of ROSC.1 The deployment of ECPR by resourced and highly trained health care teams is associated with improved survival outcomes compared to conventional CPR in specific IHCA patient populations with reversible causes.2,3 Pediatric patients with underlying cardiac disease continue to represent a population of patients with improved survival to hospital discharge and good neurologic outcomes following deployment of ECPR compared to conventional CPR for prolonged cardiac arrest.4,5 Emerging evidence suggests that risk profiles for survival differ between cardiac subpopulations (ie, surgical cardiac versus medical cardiac populations).3 Pediatric ECPR use is increasing beyond the pediatric cardiac population (eg, respiratory illness), with limited evidence suggesting benefit.2,6-8
See “Part 4: Systems of Care” for further discussion of ECPR systems of care.9
Recommendation-Specific Supportive Text
- Two single-center, retrospective case series of ECPR in patients with preexisting cardiac disease showed rates of survival to hospital discharge of 43.8% to 48%, and of survivors, 68% to 75% survived with a favorable neurologic outcome.10,11 Similar findings were reported in 5 additional single/multicenter studies among patients across illness classifications, with the majority in the cardiac illness category.12-16 A retrospective multicenter study of cardiac ICU patients from a quality improvement registry showed higher survival to discharge of ECPR recipients with cardiac surgical disease compared to cardiac medical disease,3 while analysis of 2 registries showed noncardiac diagnoses were associated with an increased risk of death following ECPR.17 A retrospective analysis from an inpatient pediatric database showed no difference in mortality between patients who received conventional CPR and patients who received ECPR, the majority of whom were cardiac surgery patients.18 Another retrospective analysis using a national registry showed higher rates of survival with ECPR in cardiac surgical patients who had a cardiac arrest.5 For noncardiac populations experiencing IHCA, a retrospective study from a national database found no difference in survival to hospital discharge between ECPR and conventional CPR.8 Additional data evaluating the use of ECPR in noncardiac patients or those who experience OHCA is insufficient to make a recommendation at this time.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. For infants and children who remain comatose following cardiac arrest, continuous central temperature monitoring is recommended. |
| 1 | B-NR | 2. For infants and children who remain comatose following cardiac arrest, avoiding central temperatures greater than 37.5 °C is recommended. |
| 2a | B-R | 3. For infants and children between 24 hours and 18 years of age who remain comatose after IHCA or OHCA, it is reasonable to use a 5-day course of targeted temperature management (TTM), either 32 °C–34 °C followed by TTM of 36 °C–37.5 °C, or only TTM of 36 °C–37.5 °C. |
Synopsis
Hypoxic ischemic brain injury is a leading cause of morbidity and mortality following pediatric cardiac arrest. Hypoxic ischemic brain injury leads to free radical production, cellular apoptosis and necrosis.1 Primary and secondary brain injury can result in cerebral ischemia or hyperemia, encephalopathy, seizures, and cerebral edema. Fever is common post–cardiac arrest and is associated with worse neurologic outcomes. Fever is also associated with increased central nervous system metabolic demands which may exacerbate primary brain injury after arrest.2,3 Targeted temperature management (TTM) refers to actively maintaining a patient’s temperature within a closely prescribed temperature range while continuously monitoring central temperature. All forms of TTM actively prevent fever. Maintaining TTM between 32 °C and 34 °C attempts to treat systemic ischemic reperfusion injury.4,5 A systematic review of pooled animal studies comparing TTM between 32 °C and 36 °C to control groups showed a strong effect of TTM on favorable neurologic outcome and reduced mortality.6
Recommendation-Specific Supportive Text
- Accurate measurement of temperature is most reliably obtained at a core site (eg, rectal, esophageal, or bladder). Continuous temperature monitoring assesses temperature swings in patients at high risk for temperature instability. Continuous core temperature monitoring was used for the 5 days of TTM in Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) trials.7,8
- Hyperthermia in the post–cardiac arrest period is common3 and is associated with decreased survival from both IHCA and OHCA. Avoiding hyperthermia offers a potential means to improve neurologic outcome.9-11 Effective prevention of fever has been shown with active cooling with servo-regulated devices compared to air-cooled devices or passive cooling techniques12 and with adoption of lower target temperature.13
- The THAPCA randomized clinical trials of TTM (32 °C–34 °C for 48 hours followed by 3 days of TTM 36 °C–37.5 °C versus TTM 36 °C–37.5 °C for a total of 5 days) after IHCA or OHCA in children with coma following ROSC found no difference in 1-year survival with a favorable neurologic outcome.7,8 Secondary analyses of the THAPCA trials showed no difference between temperature groups in any of the following subgroups: ECMO or ECPR, hypotension post-ROSC, open chest resuscitation, combined cohort of IHCA and OHCA, and acute kidney injury.14-19 A Bayesian reanalysis of the THAPCA-OH trial found a high probability that hypothermia provides a modest benefit in neurobehavioral outcome and survival at 1 year.20 A recent retrospective observational study found that children who received TTM 33 °C per clinician decision had higher health-related quality of life scores versus those who received TTM 36 °C evaluated at 3 years after the cardiac arrest.21
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. After cardiac arrest in infants and children, it is recommended to maintain systolic and mean arterial blood pressure greater than the 10th percentile for age. |
| 1 | C-EO | 2. After cardiac arrest in infants and children, continuous arterial pressure monitoring is recommended to identify and treat hypotension when appropriate resources are available. |
Synopsis
Hypotension (less than fifth percentile for age and sex) is common following ROC from cardiac arrest, occurring in 25% to 50% of infants and children.15,22 In addition to the primary causes of cardiac arrest, hypotension can be related to myocardial dysfunction and systemic reperfusion, which is associated with inflammation and vasoplegia. Hypotension can exacerbate brain ischemia and myocardial ischemic injury, systemic hypoperfusion and resultant tissue hypoxia. Myocardial dysfunction, which is often present regardless of arrest etiology, typically occurs within hours of arrest and resolves in 48 to 72 hours.23 Both the presence and severity of post arrest hypotension are associated with lower rates of survival to discharge.15,22,24 whereas normal or high blood pressures are associated with survival to discharge.14,25 When post–cardiac arrest hypotension is present, there is paucity of data regarding the association between interventions to treat hypotension and outcomes.
Recommendation-Specific Supportive Text
1 and 2. Blood pressure is often labile in the post–cardiac arrest period and recognition of hypotension is important. Continuous blood pressure monitoring allows for rapid identification of hypotension and can facilitate immediate treatment. Two observational studies associated systolic blood pressure below the fifth percentile for age in the first 12 hours following cardiac arrest with decreased rates of survival to discharge.15,22 The severity and duration of systolic hypotension within the first 72 hours post–cardiac arrest care in the pediatric ICU (PICU) are associated with decreased survival to discharge,24 whereas the combined absence of both post–cardiac arrest systolic hypotension and fever was associated with increased odds of survival to discharge.13 A secondary analysis of the ICU-Resuscitation trial of pediatric IHCA found higher rates of survival to hospital discharge as well as survival to hospital discharge with favorable neurologic outcome when blood pressure targets were above a threshold of systolic blood pressure greater than 10th percentile for age and diastolic blood pressure greater than 50th percentile for age during the first 6 hours post–cardiac arrest.26 A multicenter study of IHCA and OHCA found that mean arterial blood pressure between the fifth and 74th percentiles for age was associated with favorable neurologic outcome.27 An additional study showed that mean arterial blood pressure less than 10th percentile for age in the first 24 hours after cardiac arrest, quantified as burden of hypotension (duration and magnitude), was associated with unfavorable neurologic outcomes.28
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. After cardiac arrest in infants and children, it may be reasonable to target normoxemia that is appropriate to the specific patient’s underlying condition. |
| 2b | C-LD | 2. After cardiac arrest in infants and children, it may be reasonable to wean oxygen to target an oxyhemoglobin saturation between 94% and 99%. |
| 2b | C-LD | 3. After cardiac arrest in infants and children, it may be reasonable to target a PaCO2 that is appropriate to the specific patient’s underlying condition, and limit exposure to hypercapnia or hypocapnia. |
Synopsis
Post–cardiac arrest care is a critical component of the Chain of Survival. Monitoring of gas exchange with oxygen and ventilation titration is a key component of post–cardiac arrest care with the goal of preventing secondary end-organ injury. Although current recommendations are to administer 100% oxygen during cardiac arrest to maximize oxygenation during CPR as well as to minimize hypoxic-ischemic injury,29 target ranges for post-ROC oxygenation are less certain. Animal studies30,31 have shown associations with hyperoxia and reactive oxygen species, inflammation and brain injury, yet observational studies in infants and children post-ROC (whether spontaneous or achieved by mechanical means) have demonstrated mixed associations for hyperoxemia with survival and neurologic outcomes.32-36 While most studies demonstrated adverse outcomes with hypoxemia, 1 recent observational study did not find an association with hypoxemia in the post–cardiac arrest period.37 Associations of either duration or severity of hypoxemia or hyperoxemia with adverse outcomes in the post–cardiac arrest setting are unknown.
Extremes of arterial carbon dioxide levels lead to cerebral vasoconstriction when low (hypocapnia) and vasodilation when high (hypercapnia).38 Carbon dioxide level fluctuations and their impact on cerebral blood flow in the pediatric post–cardiac arrest population remains poorly understood, although emerging evidence suggests mild hypercapnia may be associated with survival and favorable neurologic outcome.35,37
Recommendation-Specific Supportive Text
1 and 2. Because an arterial oxyhemoglobin saturation of 100% may correspond to a Pao2 between approximately 80 mm Hg and 500 mm Hg, it is reasonable to target an oxyhemoglobin saturation between 94% and 99%. Of note, pulse oximeters may overestimate oxygen saturation levels in patients with darker skin, which can lead to lower oxygen levels (hypoxemia) going undetected.39,40 Six small observational studies of pediatric IHCA and OHCA did not show an association between hyperoxemia and outcome.32,36,37,41 One larger observational study of pediatric IHCA and OHCA, as well as a secondary analysis of the ICU-Resuscitation trial, found associations between hyperoxemia after ROC and either decreased survival to PICU discharge34 or decreased survival to hospital discharge with favorable neurologic outcome.35
3. Four observational studies found an association between hypercapnia and increased mortality and worse neurologic outcomes.33,35,37,41 Hypercapnia and hypocapnia impact cerebral blood flow. Targeting normocapnia (Paco2 35–45 mm Hg) or the patients’ baseline arterial partial pressure of carbon dioxide when chronically hypercapnic may prevent these perturbations.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. When resources are available, continuous electroencephalography (EEG) monitoring is recommended for the detection of seizures after cardiac arrest in infants and children with persistent encephalopathy. |
| 1 | C-LD | 2. It is recommended to treat clinical seizures after cardiac arrest in infants and children. |
| 2a | C-EO | 3. It is reasonable to treat nonconvulsive status epilepticus after cardiac arrest in infants and children in consultation with experts. |
Synopsis
Post–cardiac arrest brain injury remains a leading cause of morbidity and mortality in children because the brain has limited tolerance of ischemia, hyperemia, or edema. Post–cardiac arrest seizures occur in 5% to 30% of patients and can be nonconvulsive which can only be detected on electroencephalography.44,45 Post–cardiac arrest status epilepticus is associated with worse outcomes including death and neurologic injury in survivors. There are no pediatric studies assessing the efficacy of antiseizure medications for either prophylaxis or treatment of seizures and their association with outcomes such as survival to hospital discharge or survival with favorable neurologic outcome.
Recommendation-Specific Supportive Text
1. Nonconvulsive seizures and nonconvulsive status epilepticus are common after pediatric cardiac arrest and are associated with worse outcomes.44-47 The American Clinical Neurophysiology Society recommends continuous EEG monitoring for encephalopathic patients after pediatric cardiac arrest.48 Nonconvulsive seizures and nonconvulsive status epilepticus cannot be detected without EEG monitoring.48
2 and 3. There is insufficient evidence to determine whether treatment of convulsive or nonconvulsive seizures improves neurologic or functional outcomes after pediatric cardiac arrest. Both convulsive and nonconvulsive status epilepticus are associated with worse outcomes, but no study has evaluated treatment with antiseizure medications compared to no treatment.44,45 A study comparing treatment to no treatment of rhythmic and periodic discharges following adult cardiac arrest found no difference in survival or neurologic outcomes.49 The Neurocritical Care Society recommends treating status epilepticus with the goal of stopping convulsive and electrographic seizure activity.50
Figure 3 shows the checklist for post–cardiac arrest care.
Introduction
Hypoxic ischemic brain injury is the leading cause of death and disability after cardiac arrest.1 Early and reliable neurological prognostication after resuscitation from pediatric cardiac arrest is essential to guide treatment, enable accurate counseling, and provide family support. In addition, accurate neurological prognostication is critical to avoid inappropriate withdrawal of life sustaining therapy in patients who may have a meaningful recovery while also avoiding potentially inappropriate life sustaining treatments. For further discussion of ethical considerations regarding prognostication and uncertainty refer to “Part 3: Ethics.”2
The definitions of favorable and unfavorable neurological outcome are complex and dynamic throughout the recovery period after cardiac arrest. Recovery progresses along a temporal continuum such that a child assessed as having a favorable or unfavorable outcome at hospital discharge may be assessed differently several months to years after discharge. Gross scoring systems such as the PCPC are limited in the granularity of assessment and not standardized across ages, such that the same PCPC of a 6-month-old and 10-year-old may not be comparable. Thus, when classifying patients into favorable versus unfavorable outcome categories and combining timepoints of assessment, it is important to understand that these dichotomous outcomes may not give a complete picture of the individual patient’s complex reality. However incomplete the portrait of outcomes, health care professionals must still provide guidance to families, thereby necessitating criteria to classify patient outcomes.
These 2025 recommendations are based on 2 ILCOR systematic reviews of potential prognostic modalities for neurological outcome; one for prediction of good (favorable) neurological outcome and one for prediction of poor (unfavorable) neurological outcome.3,4 These reviews used the same data but analyzed them differently based on the outcome being assessed (good or poor). Definitions of good neurological outcome are not standardized and vary across studies, most often defined as a PCPC of 1 or 2; 1, 2, or 3; no change from baseline; or a Vineland Adaptive Behavioral Score of greater than 70.5,6
Good neurological outcome was assessed as a threshold false positive rate (FPR) of less than 30% (ie, predicting a good neurological outcome but having a poor neurological outcome).3,7 For poor neurological outcome, they used an FPR of less than 1% (ie, predicting a poor neurological outcome but having a good neurological outcome).4 Sensitivity (eg, probability of a good neurological outcome in a patient who has a positive finding) was assessed for all studies but was not the primary assessment of predictive accuracy. An FPR of less than 1% was selected for predictors of poor neurological outcome to minimize the risk of making recommendations for limitation of care in patients who would have a good neurological outcome. Adult studies have used FPR thresholds to predict poor neurological outcome between less than 0% and 5%.8,9 Standard thresholds to predict outcome from pediatric cardiac arrest have not been established.
The systematic review for predicting good (favorable) neurological outcome-assessed associations of positive test findings (eg, bilateral reactive pupils) with good neurological outcome, whereas the systematic review for poor neurological outcome-assessed associations of negative findings (eg, bilateral unreactive pupils) with poor neurological outcome. It cannot be presumed that positive findings for favorable outcome (ie, presence of reactive pupils with good neurological outcome) will have the same predictive value as negative findings for unfavorable neurological outcome (ie, absence of reactive pupils with poor neurological outcome). For the purposes of this guideline, we have chosen the terms favorable (good) and unfavorable (poor).
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. It is recommended that health care professionals consider multiple modalities when predicting neurological outcomes (favorable, or unfavorable after resuscitation from cardiac arrest in infants and children). |
Synopsis
Health care professionals use various assessments to guide neurological prognostication in the post–cardiac arrest period including neurological examination, biomarkers, EEG, and neurological imaging modalities (eg, brain computed tomography and magnetic resonance imaging). Studies reporting the predictive accuracy of individual assessments must be interpreted in the context of timing following ROC, outcome assessed (ie, survival versus survival with a good functional outcome), and unmeasured confounders (eg, sedation). Some modalities can predict favorable neurological outcome, unfavorable neurological outcome, or both. Each must be considered in conjunction with other modalities.
Recommendation-Specific Supportive Text
- Numerous studies demonstrate associations between clinical examination findings, biomarkers, electrophysiology patterns, and neurological imaging findings with outcomes following pediatric cardiac arrest.10-22 However, these studies are limited by their retrospective designs, lack of blinding, and unadjusted and unmeasured confounding. In addition, there are no established predictive accuracy thresholds (eg sensitivity, specificity, Area Under the Receiver Operating Curve) for individual or combined criteria. Thus, multiple modalities are needed for neurological prognostication.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. The usefulness of cough or gag reflexes or response to pain to support a favorable or unfavorable neurological prognosis at any timepoint after cardiac arrest in infants and children is not well established. |
| 2b | C-LD | 2. The usefulness of motor response to any stimulus to support a favorable or unfavorable neurological prognosis at any timepoint after cardiac arrest in infants and children is not well established. |
| 2b | C-LD | 3. The usefulness of total Glasgow Coma Scale (GCS) score or GCS motor score to support a favorable neurological prognosis at any timepoint after cardiac arrest in infants and children is not well established. |
| 2b | C-LD | 4. When interpreted in the context of other prognostic criteria, it may be reasonable to use the presence of bilateral pupillary light reflexes in the first 12 hours after cardiac arrest in infants and children to support a favorable neurological prognosis. |
| 2b | C-LD | 5. When interpreted in the context of other prognostic criteria, it may be reasonable to use the absence of bilateral pupillary light reflexes between 48 and 72 hours after cardiac arrest in infants and children to support an unfavorable neurological prognosis. |
| 3: No Benefit | B-NR | 6. The absence of pupillary light reflex in the first 24 hours after cardiac arrest in infants and children to support an unfavorable neurological prognosis is not recommended. |
| 3: No Benefit | B-NR | 7. The GCS score in the first 24 hours after cardiac arrest in infants and children to support an unfavorable neurological prognosis is not recommended. |
Synopsis
Neurologic assessments routinely include brainstem reflexes such as pupillary response, cough and gag, and motor response to stimuli. The neurologic examination evolves in the days after cardiac arrest and a given finding (eg, pupillary reactivity) at an early timepoint may not have the same accuracy for prediction of outcome at a later timepoint. Furthermore, the predictive accuracy of an exam finding (eg, pupillary reflexes) at one timepoint for favorable neurological outcome may not have the opposite predictive accuracy when the exam finding is absent (eg, absence of pupillary reflexes for unfavorable neurological outcome). Exam findings in these studies are often obtained through retrospective chart review, thus patient condition and potential confounders may not be reported which may impact the accuracy of the assessment. Clinical examination may also be confounded by sedation administration. Therefore, caution must be used when interpreting these data and health care professionals must avoid the use of isolated exam findings to predict neurological outcome.
Recommendation-Specific Supportive Text
- Three studies assessed the associations between cough or gag reflexes or evoked pain response and neurological outcome. The presence of cough and gag reflex at 24 hours predicted favorable neurological outcome with a low sensitivity of 40% for both and an FPR of 35% and 32% respectively.22,23 The absence of cough and gag predicted an unfavorable neurological outcome with an FPR of 60% and a sensitivity of 65% to 69%.23 The sensitivity for evoked pain response for favorable neurological outcome was 100%, but the FPR was as high as 67%.22,24 The absence of an evoked pain response at 6 and 12 hours to predict unfavorable neurological outcome in 1 study had an FPR of 0% (0%–15%) with a sensitivity of 33%. 22 The FPR for these assessments did not meet predefined thresholds to predict favorable or unfavorable neurological outcome.
- In a small study of 29 patients, any motor response at 48 and 72 hours after ROC had a sensitivity 80% to 100% and low FPR (23%–27%) for favorable neurological outcome.25 In that same study, the absence of any motor response to predict unfavorable neurological outcome at less than 1 hour, 48 hours, and 72 hours after ROC had a sensitivity 61% to 73%. The FPR for unfavorable neurological outcome was 62% at less than 1 hour, 20% at 48 hours and 0% at 72 hours. These data are limited to 1 small single-center study and require further evaluation.25
- Three studies including 296 patients assessed the association of mental status measured by the total GCS score greater than 4, 7 or 8, or GCS motor score greater than 4 within 24 hours of ROC with neurological outcome at ICU or hospital discharge or 6-month follow-up. They had low sensitivity (less than 50%), and low FPR (less than 14%) for favorable neurological outcome,26-28 but only 1 study assessed each timepoint and threshold and thus data were limited.
- Three studies evaluating the presence of bilateral pupillary reflexes within 12 hours of ROC demonstrated a sensitivity (ie, probability of patients with a favorable neurological outcome having bilateral pupillary response) of at least 82% for favorable neurological outcome (PCPC 1–2 or 1–3) and FPRs (ie, bilaterally reactive pupils with an unfavorable neurological outcome) of 16% to 31%.22,25,27 At timepoints greater than 24 hours from ROC, while sensitivity of bilateral pupillary reactivity was 75% to 100%, a high FPR of 68% was observed thus demonstrating its lack of reliability for prediction.23-25,28-30 Therefore, findings of bilateral pupillary reactivity greater than 24 hours post–cardiac arrest are less reliable and pupillary response alone is inadequate for neurological prognostication at any timepoint.
- Three studies evaluated the absence of pupillary reflexes to predict unfavorable neurological outcome at 48 and 72 hours after ROC with an FPR less than 1% but with wide confidence intervals (95% CI, 0%–40%) and low sensitivity of 12% to 46%.24,25,31 These FPRs met the prespecified FPR threshold and are moderately reliable, but should only be used in conjunction with other predictors.
- Six of 7 studies that assessed the absence of bilateral pupillary reflexes to predict unfavorable neurological outcome between less than 1 hour and 24 hours after ROC had an FPR greater than 10% up to 60% with sensitivities of 33% to 93%.23,25,27-30 These studies show that the absence of pupillary reflex within 24 hours after ROC are not accurate to predict outcome.
- Three studies including 296 patients assessed the association of mental status measured by the total GCS or GCS motor score within 24 hours of ROC with neurological outcome at ICU or hospital discharge or 6 month follow-up.26-28 A GCS motor score of less than 4 at 1 hour after ROC to predict unfavorable neurological outcome had high FPRs of 83% and 50%, respectively, with high sensitivities of 93% to 94%.27 A total GCS score of less than 4 at resuscitation or within 1 hour of ROC predicted unfavorable neurological outcome with a high FPR of 70% and a high sensitivity of 86%.26 A total GCS score less than 7 to predict unfavorable neurological outcome had a high FPR of 69% and high sensitivity of 92%.28 These data demonstrate that these GCS data are not accurate to predict outcome.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | B-NR | 1. When interpreted in the context of other prognostic criteria, it may be reasonable to use a plasma lactate value less than 2 mmol/L up to 12 hours after cardiac arrest in infants and children to support a favorable neurological prognosis. |
| 2b | C-LD | 2. The usefulness of neuronal biomarkers (eg, S100B, neuron-specific enolase) to support a favorable or unfavorable neurological prognosis at any timepoint after cardiac arrest in infants and children is not well established. |
| 3: No Benefit | B-NR | 3. The use of blood lactate after cardiac arrest in infants and children to support an unfavorable neurological prognosis is not recommended. |
| 3: No Benefit | B-NR | 4. The use of blood pH after cardiac arrest in infants and children to support a favorable or unfavorable neurological prognosis is not recommended. |
Synopsis
Serum biomarkers are blood-based tests that measure levels of proteins that are found in the central nervous system or measure inflammation or systemic ischemic reperfusion. Central nervous system proteins are released across the blood brain barrier when the brain is injured: Neuron-specific enolase is released from injured neurons, glial fibrillary acidic protein from injured glia, neurofilament light from injured axons, and S100B from injured astrocytes. Hypoxic ischemic brain injury and reperfusion injury may cause injury to these cells differently and release of these proteins may occur and peak at different times after injury. Studies of these neuronal biomarkers are limited by different assessment platforms, different baseline values across platforms and extracerebral sources for some proteins.
Commonly measured blood-based markers of inflammation or systemic ischemic reperfusion are pH and lactate levels. Lactate levels may reflect the severity of systemic hypoxia and ischemia before, during, or after cardiac arrest. pH is impacted by both systemic acidosis, of which lactate acidosis may be a major contributor, as well as respiratory acidosis, which may be due to inadequate ventilation. These systemic markers change over time after arrest. pH can be modified by titration of the ventilator to correct respiratory acidosis as well as administration of sodium bicarbonate to treat metabolic acidosis. Lactate clearance refers to the decrease in lactate over time after cardiac arrest; earlier clearance is associated with decreased mortality in adults.32
Recommendation-Specific Supportive Text
- Serum lactate less than 2 mmol/L within 12 hours of ROC had low sensitivity of 16% to 28% for favorable neurological outcome, but a very low FPR ranging from 4% to 7%.33-35 Lactate thresholds of less than 2 mmol/L at 24 and 48 hours and less than 5 mmol/L at 1 and 24 hours had high FPRs of 17% to 68% and were moderately sensitive (61%–89%).33,36 Thus only timepoints before 12 hours should be considered to predict outcomes in conjunction with other findings.
- Neuronal biomarkers S100B, neuron-specific enolase, myelin basic protein , neurofilament light, ubiquitin C-terminal hydrolase-L1, glial fibrillary acidic protein and tau have been assessed at timepoints ranging from less than 1 hour through 96 hours after ROC.20,29,37,38 Various thresholds were assessed for biomarkers to predict favorable neurological outcome at different timepoints resulting in a very wide ranges of FPR (0% to 96%) and sensitivities (5% to 100%). Three studies of various thresholds for S100B had an FPR of 0% with sensitivities of 29% to 38% for unfavorable neurological outcome and for neuron-specific enolase had FPR of 0% with sensitivities of 19% to 26%.20,34,45 While these FPRs for favorable outcome were low, there were no consistent thresholds evaluated for these markers. Further study is needed to validate specific biomarker thresholds and discrete timepoints.
- Lactate was assessed in 6 studies.20,34,35,39-41 Two studies had an FPR of less than 1% for unfavorable neurological outcome, 1 with a sensitivity of 11% with a threshold greater than 28.8 mmol/L 1 hour after ROC and 1 with a lactate clearance less than 2 mmol/L by 48 hours post-ROC.20,39 All other studies had FPR ranging from 11% to 83% for unfavorable neurological outcome.33-35,41
- pH level was assessed in 4 studies.20,34,35,39 A pH threshold of greater than 7.0 at 1 hour and 6 to 12 hours post–cardiac arrest had high sensitivities of 71% to 96%, but high FPRs ranging from 45% to 97% for favorable neurological outcome. A pH threshold of greater than 7.3 at 1 hour and 24 hours post–cardiac arrest had sensitivities of 49% and 89% respectively but FPRs of 38% and 81% respectively for favorable neurological outcome.40 For unfavorable neurological outcome, 3 studies had FPRs greater than 5%, but sensitivities of 3% to 14%.34,35 pH is not accurate enough to predict favorable or unfavorable neurological outcome.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. When interpreted in the context of other prognostic criteria, it is reasonable to use EEG up to 72 hours after cardiac arrest in infants and children to support a favorable or unfavorable neurological prognosis. |
| 2b | B-NR | 2. When interpreted in the context of other prognostic criteria, it may be reasonable to use the presence of continuous or normal EEG background in the first 72 hours after cardiac arrest in infants and children to support a favorable neurological prognosis. |
| 2b | C-LD | 3. When interpreted in the context of other prognostic criteria, it may be reasonable to use the presence of sleep spindles or stage II sleep architecture on EEG between 12 and 24 hours after cardiac arrest in infants and children to support a favorable neurological prognosis. |
| 2b | C-LD | 4. When interpreted in the context of other prognostic criteria, it may be reasonable to use the presence of EEG reactivity between 6 and 24 hours after cardiac arrest in infants and children to support a favorable neurological prognosis. |
| 2b | C-LD | 5. When interpreted in the context of other prognostic criteria, it may be reasonable to use the presence of status epilepticus, or the presence of burst suppression, burst attenuation or generalized periodic epileptiform discharges between 24 and 72 hours after cardiac arrest in infants and children to support an unfavorable neurological prognosis. |
| 2b | C-LD | 6. The usefulness of the presence of attenuated, isoelectric or flat EEG, or presence of myoclonic status epilepticus at any timepoint after cardiac arrest in infants and children to support an unfavorable neurological prognosis is not well established. |
| 3: No Benefit | B-NR | 7. The presence of clinical or electrographic seizures, absence of sleep spindles and stage II sleep architecture, absence of continuous or normal EEG background, absence of EEG reactivity and absence of EEG variability at any timepoint after cardiac arrest in infants and children is not recommended to support an unfavorable neurological prognosis. |
| 3: No Benefit | B-NR | 8. The absence of burst suppression, burst attenuation, generalized periodic epileptiform discharges, attenuated, isoelectric, or flat EEG at any timepoint after cardiac arrest in infants and children is not recommended to support a favorable neurological prognosis. |
| 3: No Benefit | B-NR | 9. The absence of clinical or electrographic seizures, absence of status epilepticus, or absence of myoclonic seizures at any timepoint after cardiac arrest in infants and children is not recommended to support a favorable neurological prognosis. |
Synopsis
EEG is broadly used to monitor for subclinical seizures and to assess background states after brain injury. Patients who are encephalopathic after cardiac arrest may have subclinical seizures due to cortical injury which cannot be detected without EEG monitoring. Electroencephalography for neurological prognostication has promise as it directly assesses neurologic activity. However, studies are limited because most are single center, utilize nonstandardized terminology, lack clinician blinding to EEG data when caring for patients, and do not describe the impact of medications on EEG background. These systematic reviews assessed multiple EEG features including the presence and absence of normal and abnormal EEG features with favorable and unfavorable neurological outcomes.5,7
Recommendation-Specific Supportive Text
- EEG monitoring up to 72 hours after ROC identifies EEG background features and diagnoses electrographic seizures. In patients who are encephalopathic, clinical exams can be unreliable and seizures can be subclinical. Electroencephalography is the only way to identify subclinical seizures.21,22,30,42-46
- Ten studies with more than 560 patients evaluated the association of either continuous or routine (brief) EEG at either less than 1 hour; 6 to 12 hours; 24, 48,72 hours; or 4 to 6 days with favorable neurological outcome at PICU or hospital discharge or 6 months following cardiac arrest. Among the studies evaluating EEG performed at 6 to 12 and 24 hours post-ROC, 6 of 7 studies had FPRs less than 29% for favorable neurological outcome with sensitivities that ranged from 7% to 100%.23,30,42,44,45,47,48 Studies at 48 and 72 hours reported FPRs ranging from 0% to 50% for favorable neurological outcome, although 50% of the studied timepoints had FPRs less than 30%.21,24,29,47
- The presence of sleep architecture at 6 to 12 hours or sleep spindles at 24 hours had an FPR of 16% and 8% for favorable neurological outcome, with sensitivities of 57% and 80%, respectively. Sleep spindles or sleep II architecture should not be used as the sole assessment to prognosticate at any timepoint.23,30
- At 6 to 12 and 24 hours post-ROC, the presence of reactivity had an FPR of less than 27% with a sensitivity of 53% to 63% for favorable neurological outcome.42,44 At 48 hours, 1 study had an FPR of 50%.44 Thus EEG reactivity, based on these data, is accurate only at 6 to 24 hours, not 48 hours for favorable neurological outcome.
- The presence of status epilepticus predicted unfavorable neurological outcomes with a low FPR of 0% at 24 to 72 hours after ROC in 3 studies,21,24,48 but an FPR of 4% to 5% at 4 to 6 hours and 6 to 12 hours, all with low sensitivity.42,45 Thus, the presence of status epilepticus at 24 to 72 hours is moderately predictive for unfavorable neurological outcome. The presence of burst suppression, burst attenuation or generalized periodic epileptiform discharges within 24 hours of ROC had an FPR that ranged from 0% to 19% and sensitivity of 9% to 30% for unfavorable neurological outcome.23,44,45,49 From 48 to 72 hours after ROC, 3 studies had FPRs less than 1% (95% CI, upper limit range 16%–54%) with sensitivities of 0% to 67% for unfavorable neurological outcome.21,22,24 The prediction of unfavorable neurological outcome was moderately reliable from 24 to 72 hours.
- Two studies of 61 patients, of which 8 had myoclonic status epilepticus, had an FPR of 0% (95% CI, 0%–34%) and sensitivity of 17% to 21% at PICU/hospital discharge.22,44 The presence of attenuated, isoelectric, or flat EEG before 24 hours in 5 studies had FPRs ranging from 6% to 95%,23,42,45,47,49 except for 2 studies that had and FPR of 0% with upper limit confidence intervals of 4% to 31%.30,50 In 6 studies from 48 hours to 7 days, 2 had FPRs less than 1% with 95% CI up to 34% to 52%22,44; while other studies had FPRs that ranged from 3% to 71%.21,29,47,51 There are conflicted data and further study is needed.
- Of 10 studies, the presence of seizures between 4 to 6 hours and 24 hours post-ROC had an FPR of 0% to 20%, of which only 1 had an FPR of less than 1% with wide confidence intervals44,45,50 and a sensitivity of 2% to 38% for predicting unfavorable neurological outcome.27,30,34,42,44,45,48-50 At 48 hours and onward only 2 studies reported an FPR for predicting unfavorable outcome of less than 1%41,44; others had FPRs up to 58%. The presence of seizures is accurate to predict unfavorable neurologic outcome at any of these timepoints. The absence of a normal/continuous EEG background patterns (defined as normal, continuous, and reactive; continuous and unreactive; and nearly continuous) by ACNS definitions52 had variability and high FPRs ranging from 0% to 90%21-24,29,30,42,44,45,47-50; only 2 studies had an FPR less than 1%.48,51 The absence of a normal/continuous EEG background pattern is not accurate to predict unfavorable neurological outcome. The absence of EEG reactivity had an FPR of 0% to 93% and sensitivity of 36% to 100%21,42,44; absence of sleep II architecture had an FPR 2 of 0% to 43% and sensitivity of 84% to 92%23,30,42;and absence of variability on EEG42,44 had an FPR of 0% to 80% and sensitivity of 21% to 82% for unfavorable neurological outcome prediction. These were unreliable tests for unfavorable outcome prediction.
- Absence of attenuated, isoelectric or flat EEG to predict favorable neurological outcome was assessed in 10 studies.21-23,29,30,42,44,45,47,51 The FPR was greater than 40% in most studies and the sensitivity was high in most studies at all timepoints (71%–100%).23,30,42,44,45,47,51 While the absence of burst suppression, burst attenuation, or generalized periodic epileptiform discharges had a sensitivity for favorable neurological outcome of greater than 81% at all timepoints, the FPR was greater than 67% at all timepoints.21-23,42,44,45 These EEG features should not be used to predict favorable neurological outcome.
- The absence of seizures had FPRs of 58% to 100% and sensitivities that ranged from 50% to 100% for favorable neurological outcome at PICU or hospital discharge, or 6 or 12 months post–cardiac arrest.21,22,27,30,34,35,42,44,45,51 Absence of status epilepticus at 6 to 12 or 72 hours had a high sensitivity (greater than 96%) for favorable neurological outcome, but very high FPRs (75% to 91%).21,24,42,45 The absence of myoclonus in the 48 hours post–cardiac arrest was 100% sensitive for favorable neurological outcome but had high FPRs (79% to 83%).22,44 Thus these EEG features should not be used to predict favorable neurological outcome at any timepoint.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | B-NR | 1. When interpreted in the context of other prognostic criteria, it may be reasonable to use a normal brain magnetic resonance imaging (MRI) between 72 hours and 2 weeks after cardiac arrest in infants and children to support a favorable neurological prognosis. |
| 2b | C-LD | 2. When interpreted in the context of other prognostic criteria, it may be reasonable to use an abnormal brain MRI showing high ischemic burden at 72 hours or later after cardiac arrest in infants and children to support an unfavorable neurological prognosis. |
| 2b | C-LD | 3. When interpreted in the context of other prognostic criteria, it may be reasonable to use a CT with loss of gray-white matter differentiation within 24 hours after cardiac arrest in infants and children to support an unfavorable neurological prognosis. |
| 3: No Benefit | C-LD | 4. A normal brain CT scan within the first 48 hours after cardiac arrest in infants and children is not recommended to support a favorable neurological prognosis. |
Synopsis
Early neuroimaging after cardiac arrest is useful to detect structural brain injury and potential etiologies of cardiac arrest that may be amenable to intervention such as evacuation of intracranial hemorrhage. MRI can assess the severity of cytotoxic injury through diffusion weighted imaging. Ischemic injury on diffusion weighted imaging peaks at days 3 to 7 after cardiac arrest and may have the appearance of normalizing, or “pseudonormalization,” 2 weeks after injury. Both computed tomography (CT) and MRI findings evolve over the days after cardiac arrest and must be interpreted in the context of other findings and based on time from injury. Studies assessing neuroimaging for the prediction of neurological outcome require careful interpretation as they are limited by retrospective designs where health care professionals have likely used imaging results to guide treatment decisions.
Recommendation-Specific Supportive Text
- In 3 studies, absence of diffusion restriction or absence of any abnormality on MRI had a sensitivity of 42% to 88% with FPRs of 0% to 2% for favorable neurological outcome.24,51,53 Apparent diffusion coefficient thresholds of greater than 600×10–6 mm2/s in greater than 93% and greater than 650×10–6mm2/s in greater than 89% of brain volume, at a median of 4 days after ROC, predicted favorable neurological outcome with a sensitivity of 100% and low FPR of 20%.54 While the absence of specific regional abnormalities on MRIs were highly sensitive for favorable neurological outcome, they had very high FPRs.31,55
- In 3 studies, the presence of high burden of ischemia defined as apparent diffusion coefficient threshold less than 650×10–6 mm2/s in ≥10% of brain volume at a median of 4 days after ROC, predicted unfavorable neurological outcome with a sensitivity of 49% to 52% and an FPR of 0% to 6% (95% CI, 1%–21%)49,54,56 but only 1 had an FPR less than 1% with a sensitivity of 80%.54 The presence of diffusion restriction based in any brain region based on nonstandardized definitions at a median of 4 days and up to 14 days had high FPR (12%–58%) for unfavorable neurological outcome.24,56 The studies found that the abnormalities in diffusion weighted imaging, T1-, and T2- weighted imaging in individual regions of the brain, at 4 to 6 days post-ROC, predicted unfavorable outcome with FPR of 0% to 10% but wide confidence intervals up to 50%.24,31,55
- One study of 78 children had an FPR 0% (CI 0%–12%) and sensitivity of 65% for the loss of gray-white matter differentiation on CT within 24 hours of arrest to predict unfavorable neurological outcome (PCPC greater than 3) at hospital discharge.16 Clinicians were not blinded to the CT results in any study.
- In 2 studies, the absence of grey-white matter differentiation on CT within 24 hours from ROC had a sensitivity of 64% to 100% with an FPR between 35% and 75% for favorable neurological outcomes at hospital discharge.16,57 Absence of a reversal sign and cistern or sulcal effacement had high FPRs that ranged from 14% to 80% sensitivities despite sensitivities that ranged from 93% to 100%.16,57 Normal CT early after cardiac arrest is not accurate to predict favorable neurologic outcome.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. It is recommended that infants and children who survive cardiac arrest be evaluated for rehabilitation services. |
| 2a | B-NR | 2. It is reasonable that infants and children who survive cardiac arrest be evaluated for physical, cognitive, and emotional needs to guide follow-up care within the first year following cardiac arrest. |
Synopsis
Survivors of both in-hospital and out-of-hospital cardiac arrest are at significant risk for both short-term and long-term physical, neurological, cognitive, emotional, and social morbidities.1-3 Children who survive a cardiac arrest with a grossly favorable outcome on the PCPC scale may have more subtle and sustained neuropsychological impairment.4 The full impact of brain injury on children’s development may not be fully appreciated until months to years after the cardiac arrest and discharge outcome does not necessarily predict long-term outcome, as some survivors with unfavorable outcome improve and others with favorable outcome at discharge worsen over time.5 Recovery and Survivorship is the sixth ring in the Chain of Survival and acknowledges that survivors of cardiac arrest may require ongoing integrated medical, rehabilitative, caregiver, and community support in the months to years after their cardiac arrest (see Figure 5).6 The overall societal burden of pediatric OHCA was assessed from 2016 to 2020, in terms of disability-adjusted life years as a common public health metric to estimate burden of disease, placing nontraumatic OHCA as 1 of the top 10 leading causes of annual disability-adjusted life years.7
Recommendation-Specific Supportive Text
- Two randomized controlled trials of TTM for comatose children after IHCA or OHCA, with a primary outcome of favorable neurobehavioral function defined by the Vineland Adaptive Behavioral Score at 1 year post–cardiac arrest, showed that new morbidity in survivors is common.8,9 Many children who survived to 1 year with a favorable neurobehavioral outcome on Vineland Adaptive Behavior Scales-II had global cognitive impairment or selective neuropsychological deficits.10
- Recent statements from the AHA highlight the importance of follow-up after discharge.1,11 Several case series of pediatric cardiac arrest outcomes at greater than 1 year post–cardiac arrest demonstrate ongoing cognitive, physical, and neuropsychological impairments.2-5 Longitudinal follow-up studies up to 5 years after cardiac arrest identified cognitive and behavioral deficits in pediatric survivors who had absent or minimal neurologic change from prearrest baseline at hospital discharge.5,10 Compared to healthy controls, survivors of cardiac arrest have specific cognitive deficits correlated with structural brain abnormalities demonstrated on MRI.12 Patients discharged with functional impairments often face years of impaired function and significant health care needs. The impact of ongoing childhood development on recovery following pediatric cardiac arrest is unknown.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Family members should be provided the option of being present during the resuscitation of their infant or child. |
| 1 | B-NR | 2. When family members are present during resuscitation of their infant or child, it is recommended for a designated team member to provide comfort, answer questions, and support the family. |
| 1 | C-LD | 3. If the presence of family members is considered detrimental to the resuscitation of the infant or child, family members should be respectfully asked to leave. |
Synopsis
The practice of allowing family presence during resuscitation has become more common over the last 25 years.1 Parents may desire to be present during their child’s resuscitation to provide emotional support, share information with the care team,2 and observe resuscitation measures. Parental presence may offer a sense of involvement, reduce helplessness, and provide closure.3 Presence at their child’s death can lead to lower rates of anxiety and depression as well as improved grieving behaviors such as social withdrawal and changes in activity levels. 4,5
Health care professionals’ perspectives on family presence differ significantly, but acceptance generally rises with prior experience and among senior professionals.3 Parental presence can be encouraged by providing parents with adequate support and consistent updates throughout the resuscitation process, benefiting both the family and the health care team. While health care professionals often worry about the potential negative psychological effects on families, evidence suggests benefit for families to be present during resuscitation of their child.
Recommendation-Specific Supportive Text
- A systematic review of qualitative survey-based studies reported that 85% of parents surveyed expressed a desire to be present during their child’s resuscitation.6 Additionally, parents of 272 children who underwent resuscitation perceived benefits from their presence, such as reduced anxiety and improved understanding and processing of the event.6 The presence of family members can enhance parental satisfaction with the care provided, provide families with a greater sense of control,7 and improve self-reported adjustment to the loss of their child.3,8 Additionally, parent surveys indicated a strong desire to be present to understand what was happening, ensure that all possible measures were being taken, and maintain physical contact with their child.3,9,10 Not all parents who have witnessed their child’s resuscitation would choose to do so again.11 While concerns have been raised regarding family presence during resuscitation including potential trauma for the family, interference with procedures, effects on technical performance, and challenges for teaching and clinical decision-making, these concerns have not been substantiated by current evidence.7,12-14
- The presence of a facilitator to support families is beneficial and helps reduce stress.15-19 One study reported that 72% of health care professionals support the use of a dedicated staff member for this role during resuscitation.20 A systematic review emphasized the significance of family facilitators in providing emotional support and reducing parental anxiety during resuscitation.21 Team members serving as communication liaisons have been reported to update families in real-time, explain medical terminology, and ensure effective two-way communication. Both parents and health care professionals emphasized the importance of training personnel to support family presence.22,23 A qualitative study highlighted essential competencies for facilitators, such as the ability to provide active listening, foster partnership, and facilitate communication between the family and resuscitation team leader.20 In addition, 2 systematic reviews showed that parents often seek spiritual and cultural comfort during pediatric resuscitations.19,21 Including spiritual healthcare professionals /chaplains can help align clinical care with families’ needs, enhance the emotional safety of resuscitations, and support long-term healing.24 While having a dedicated team member to support families during resuscitations can help in the processing of traumatic events, this may not always be feasible and the absence of such a team member does not preclude family presence during resuscitation.
- Most surveys suggest that family presence during resuscitation is not disruptive, although some health care professionals report increased stress.25 Health care professionals with extensive experience in family presence recognize that occasional negative experiences may occur.26 Concerns have been raised by both health care professionals and family members that parental presence during resuscitation may be distracting and could impact the resuscitation. Health care professionals, in particular, often worry about the risk of litigation and the potential negative psychological effects on families.3,7 One randomized trial found that witnessed resuscitation increased health care professionals’ anxiety yet reduced anxiety in family members.27 In randomized controlled simulation studies, family presence did not affect hands-on time or CPR quality, but led to increased frustration, perceived temporal demands, and mental demands on health care professionals.28,29
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. A complete unrestricted autopsy of infants and children with sudden unexplained death is recommended, preferably performed by a pathologist with training and experience in cardiovascular pathology with preservation of biological material for further genetic analysis to determine the presence of inherited cardiac disease. |
| 1 | B-NR | 2. When conventional autopsy does not identify a cause of death or diagnosis in infants and children with sudden unexplained death, a postmortem genetic evaluation is recommended, when resources allow, especially if clinical evidence suggests the diagnosis of an inherited cardiac disease. |
| 1 | B-NR | 3. When autopsy does not identify a cause of death or is not performed in infants and children with sudden unexplained death, it is recommended that first degree family members be referred to a health care professional or center with expertise in inherited cardiac disease and cardiac genetic counseling. |
| 1 | C-EO | 4. For infants and children who survive sudden unexplained cardiac arrest, obtain a complete past medical and family history (including a history of syncopal episodes, seizures, unexplained accidents or drowning, or sudden unexpected death before 50 years of age), review previous electrocardiograms, and refer to a cardiologist with expertise in inherited cardiac disease. |
Synopsis
Inherited cardiac diseases (ie, cardiomyopathies, channelopathies), and coronary artery anomalies are common causes of sudden unexplained cardiac arrest in infants and children. In patients who do not survive, autopsy plays an essential role to correctly identify the etiology of the cardiac arrest. Up to one third of young patients who do not survive sudden unexplained cardiac arrest have no abnormalities found on gross and microscopic autopsies.1-4 Postmortem genetic evaluation (“molecular autopsy”) is increasingly used as an important tool to evaluate potential causes of sudden unexplained death and identify inheritable cardiac diseases.5 In addition to providing an explanation, genetic diagnosis can enable necessary screening and preventive measures for relatives and survivors.
Recommendation-Specific Supportive Text
- Previous reviews and consensus guidelines state the importance of an autopsy following sudden unexplained cardiac death to aid in determining the cause of death and to identify possible inherited cardiac conditions.6-9 An autopsy can identify several causes of death in infants and children with sudden unexplained cardiac death including cardiomyopathy (ie, hypertrophic, dilated, or arrhythmogenic) and anomalous coronary arteries. When no cause of death is identified on gross examination, microscopic evaluation and preservation of biological tissue is paramount to be able to perform a molecular autopsy if resources allow.
- Previous systematic reviews and consensus guidelines emphasize the use of postmortem genetic testing for inherited cardiac causes of sudden death (ie, cardiomyopathies, channelopathies). A review reported up to 50% of sudden cardiac death cases in infants and children had a potential genetic disease identified.7 In multiple studies, genetic mutations causing channelopathies were identified in 2% to 10% of infants with sudden infant death syndrome.10-16 Among children with sudden unexplained death and a normal autopsy, 9 cohort studies report identification of genetic mutations associated with channelopathy or cardiomyopathy.14,17-24
- In multiple studies, 13% to 53% of first- and second-degree relatives of patients with unexplained cardiac arrest were diagnosed with inherited cardiac disease after screening using medical and family history, clinical exam, and laboratory evaluation.7,17-20,25-32 A small case series suggested that genetic screening of family members should be directed by clinical history.17
- A retrospective study of 155 adult survivors of nonischemic sudden cardiac arrest reported 49% of survivors were diagnosed with an inherited cardiac condition.27 Several cohort studies report the utility of obtaining a complete past medical and family history, including multigenerational pedigree, after sudden unexplained cardiac arrest as well as review of prior electrocardiograms. Three small cohort studies and 1 population-based study reported relevant clinical symptoms or medical comorbidities before cardiac arrest, such as seizure, syncope, palpitations, chest pain, left arm pain, and shortness of breath, among patients who had a sudden unexplained cardiac arrest.18,19,21,22
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Health care professionals should reassess infants and children after every fluid bolus to assess for fluid responsiveness and signs of volume overload. |
| 2a | B-R | 2. Either isotonic crystalloids or colloids can be effective as the initial fluid choice for resuscitation in infants and children. |
| 2a | B-NR | 3. Either balanced or unbalanced solutions can be effective as the fluid choice for resuscitation in infants and children. |
| 2a | C-LD | 4. In infants and children with septic shock, it is reasonable to administer fluid in 10-mL/kg or 20-mL/kg aliquots with frequent reassessment. |
| 2a | C-LD | 5. In infants and children with fluid-refractory septic shock, it is reasonable to use either epinephrine or norepinephrine as an initial vasoactive infusion. |
| 2a | C-EO | 6. For infants and children with cardiac arrest and sepsis, it is reasonable to apply the standard PALS algorithm compared with any unique approach for sepsis-associated cardiac arrest. |
| 2b | B-NR | 7. For infants and children with septic shock unresponsive to fluids and requiring vasoactive support, it may be reasonable to consider stress-dose corticosteroids. |
| 2b | C-LD | 8. In infants and children with fluid-refractory septic shock, if epinephrine or norepinephrine are unavailable, dopamine may be considered. |
Synopsis
Shock is the failure of oxygen delivery to meet tissue metabolic demands and can be life threatening. The most common type of pediatric shock is hypovolemic, including hemorrhagic shock, with distributive, cardiogenic, and obstructive shock occurring less frequently. Multiple types of shock can occur simultaneously, and early presentations can be subtle; health care professionals must be vigilant.
Shock progresses over a continuum of severity, from a compensated to a decompensated (hypotensive) state. Compensatory mechanisms include tachycardia and increased systemic vascular resistance to maintain cardiac output and end-organ perfusion. As compensatory mechanisms fail, hypotension and signs of inadequate end-organ perfusion develop, such as depressed mental status, decreased urine output, acidosis, and weak central pulses.
Sepsis is a life-threatening response to infection, where the body’s immune system overreacts, leading to widespread inflammation and organ dysfunction. Septic shock is characterized as sepsis with cardiovascular dysfunction including hypotension. Early recognition and prompt treatment (eg, antibiotics, fluids) are critical for improving outcomes in infants and children. Mortality from pediatric sepsis has declined, concurrent with implementation of guidelines emphasizing early antibiotic and fluid administration.1 Controversies in septic shock management include volume and type of fluid administration, vasopressor timing and choice, and use of corticosteroids. Previous AHA guidelines2 had considered large studies of patients with malaria, sickle cell anemia, and dengue shock syndrome; however, generalization of results from these studies is problematic.
This topic was last reviewed in the 2020 AHA Guidelines for CPR and ECC. These recommendations have not been updated for this edition of the Guidelines, with the exception of No. 6, which was reviewed and updated.3
Recommendation-Specific Supportive Text
- Although fluids remain the mainstay initial therapy for infants and children in shock, especially in hypovolemic and septic shock, fluid overload can lead to increased morbidity.4 In 2 randomized trials of patients with septic shock, those who received higher fluid volumes5 or faster fluid resuscitation6 were more likely to develop clinically significant fluid overload characterized by increased rates of mechanical ventilation and worsening oxygenation.
- In a systematic review, 12 relevant studies were identified, though 11 assessed colloid or crystalloid fluid resuscitation in patients with malaria, dengue shock syndrome, or “febrile illness” in sub-Saharan Africa.7 There was no clear benefit to crystalloid or colloid solutions as first-line fluid therapy in any of the identified studies.
- One randomized controlled trial compared the use of balanced (lactated Ringer’s solution) to unbalanced (0.9% saline) crystalloid solutions as the initial resuscitation fluid and showed no difference in relevant clinical outcomes.8 A matched retrospective cohort study of pediatric patients with septic shock showed no difference in outcomes,9 though a propensity-matched database study showed an association with increased 72-hour mortality and vasoactive infusion days with unbalanced crystalloid fluid resuscitation.10
- In a small, randomized controlled study, there were no significant differences in outcomes with the use of 20 mL/kg as the initial fluid bolus volume in septic shock, compared to 10 mL/kg; however, the study was limited by a small sample size.5
- Two pediatric randomized controlled trials comparing escalating doses of dopamine or epinephrine demonstrated improvement in timing of resolution of septic shock11 and 28-day mortality12 with the use of epinephrine over dopamine. Both studies were conducted in resource-limited settings, and the doses of inotropes used may not have been directly comparable, limiting conclusions from the studies. Medications that increase systemic vascular resistance, such as norepinephrine, may also be a reasonable initial vasopressor therapy in septic shock patients.1,13-15 International sepsis guidelines recommend the choice of medications to be guided by patient physiology and clinician preferences.1
- No studies support deviations from standard life-support algorithms to improve outcomes in patients with sepsis-associated cardiac arrest. Sepsis-associated cardiac arrest is associated with worse outcomes than other causes of cardiac arrest.16
- A meta-analysis17 showed no change in survival with corticosteroid use in pediatric septic shock, though a subsequent randomized controlled trial suggested a shorter time to reversal of shock with steroid use.18 Two observational studies19,20 suggested there may be specific subpopulations, based on genomics, that would either benefit or experience harm from steroid administration, though these subpopulations are difficult to identify clinically. Patients at risk for adrenal insufficiency (eg, those on chronic steroids, patients with purpura fulminans) are more likely to benefit from steroid therapy.14
- In situations when epinephrine or norepinephrine are not available, dopamine is a reasonable alternative initial vasoactive infusion in patients with fluid-refractory septic shock.11,12 Patients with vasodilatory shock may require a higher dose of dopamine.13
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For infants and children with cardiogenic shock, early expert consultation is recommended. |
| 2b | C-EO | 2. For infants and children with cardiogenic shock, it may be reasonable to use epinephrine, dopamine, dobutamine, or milrinone as an inotropic infusion. |
Synopsis
Cardiogenic shock occurs when the heart is unable to pump blood efficiently, resulting in decreased cardiac output and inadequate oxygen delivery to tissues. While it is relatively rare in children compared to other forms of shock, it is associated with high rates of mortality.21 Causes of cardiogenic shock include congenital heart disease, myocarditis, cardiomyopathies, and arrythmias. Clinical manifestations vary by severity and underlying cause. Initial compensatory mechanisms include tachycardia, tachypnea, and increased systemic vascular resistance. As compensatory mechanisms fail, hypotension and signs of inadequate end-organ perfusion develop, such as depressed mental status, decreased urine output, lactic acidosis, and weak central pulses. Initial treatment is focused on improving oxygen delivery, minimizing oxygen consumption, and restoring cardiac function through administration of vasoactive medications. It is important to note that cardiogenic shock can occur with other forms of shock simultaneously. In its early stages, it can be difficult to diagnose, so a high index of suspicion is warranted. This topic was last reviewed in the 2020 AHA Guidelines for CPR and ECC. These recommendations have not been updated for this edition of the Guidelines.3
Recommendation-Specific Supportive Text
1 and 2. Cardiogenic shock in infants and children is uncommon and associated with high mortality rates. No studies were identified comparing outcomes between vasoactive medications. For patients with hypotension, vasoactive medications such as epinephrine may be more appropriate as an initial inotropic therapy. Because of the rarity and complexity of these presentations, expert consultation is recommended when managing infants and children in cardiogenic shock.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO | 1. Among infants and children with hypotensive hemorrhagic shock following trauma, it is reasonable to administer blood products, when available, instead of crystalloid for ongoing volume resuscitation. |
Synopsis
Resuscitation guidance for children with hemorrhagic shock is evolving, as crystalloid-then-blood paradigms are being challenged by resuscitation protocols using blood products early in resuscitation. However, the ideal resuscitation strategy for a given type of injury is often unknown.
This topic was last reviewed in the 2020 AHA Guidelines for CPR and ECC. These recommendations have not been updated for this edition of the Guidelines.3
Recommendation-Specific Supportive Text
- There are no prospective pediatric data comparing the administration of early blood products versus early crystalloid for traumatic hemorrhagic shock. A scoping review identified 6 retrospective studies that compared patient outcomes with the total volume of crystalloid resuscitation received in the first 24 to 48 hours among children with hemorrhagic shock.22-27 Four studies reported no differences in survival to 24 hours, survival at 30 days with good neurological outcome, or survival to discharge.22,24-26,28 Large-volume resuscitation was associated with increased hospital/ICU length of stay in 5 of the 6 studies.23-28 One study reported lower survival to hospital discharge among children who received more than 60 mL/kg crystalloid compared to lower volume groups.27 Despite limited pediatric data, guidelines for adults from the Eastern Association for the Surgery of Trauma,29 the American College of Surgeons, and the National Institute for Health and Care Excellence30 suggest the early use of balanced ratios of packed red blood cells, fresh frozen plasma, and platelets for trauma-related hemorrhagic shock.29 The American College of Surgeons, and the National Institute for Health and Care Excellence30 suggest the early use of balanced ratios of packed red blood cells, fresh frozen plasma, and platelets for trauma-related hemorrhagic shock.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. When a cuffed endotracheal tube (ETT) is used in infants and children, attention should be paid to ETT size, position, and cuff inflation pressure (usually less than 20–25 cm H2O). |
| 2a | B-NR | 2. It is reasonable to choose cuffed ETTs over uncuffed ETTs for intubating infants and children. |
Synopsis
Cuffed and uncuffed ETTs have been studied in the perioperative period and up to 48 hours postintubation. These studies have shown that cuffed ETTs are safe in infants and children and are not associated with significant differences in postextubation airway complications.1-6 The primary advantage of cuffed ETTs over uncuffed tubes is a reduction in the need for ETT changes, which reduces high-risk reintubations and interruptions in chest compressions during CPR that may occur during reintubation. Additional potential benefits include improved capnography accuracy and a lower risk of atelectasis.2,5-13 Although several studies have identified that cuffed tubes may decrease airway trauma by decreasing tube exchanges, attention must be directed to selecting the correct tube size3 and cuff inflation pressure.14 ETT cuff pressures are dynamic during transport at altitude,15 and with increasing airway edema or pressure, make it necessary to monitor the cuff pressure to avoid damage to the airway mucosa.
Recommendation-Specific Supportive Text
- A retrospective study of 155 neonates and children under 12 years of age undergoing cardiac surgery with cardiopulmonary bypass and intubated for at least 4 hours postoperatively found larger ETT size as the only significant factor associated with airway complications.3 A retrospective study of 2,953 children found that applying 25 cm H2O of cuff pressure with a slight leak around the ETT resulted in no cases of clinically significant subglottic stenosis and the incidence of reintubation for stridor was less than 1%.1
- Three systematic reviews, 5 randomized controlled trials, and 3 retrospective reviews support the safety of cuffed ETTs in infants and children.2-5,7-13 While these studies were almost entirely performed in the perioperative patient population, 2 studies included patients who remained intubated for 4 hours3 or over 12 hours postoperatively.5 An additional retrospective review evaluating the use of perioperative cuffed ETTs in 1162 infants weighing 2 to 5 kg demonstrated no increase in postoperative airway complications.6 The use of cuffed ETTs is associated with lower reintubation rates, more successful ventilation, and improved accuracy of capnography without increasing the risk of complications.2,5-9,11-13,16-18 Cuffed ETTs may also decrease the risk of aspiration and atelectasis.5,19,20
| COR | LOE | Recommendations |
|---|---|---|
| 3: No Benefit | C-LD | 1. Routine use of cricoid pressure to reduce the risk of regurgitation is not recommended during bag-mask ventilation of infants and children. |
| 3: No Benefit | C-LD | 2. Routine use of cricoid pressure is not recommended during tracheal intubation of infants and children. |
| 3: Harm | C-LD | 3. If cricoid pressure is used, discontinue if it interferes with ventilation or the speed or ease of tracheal intubation in infants and children. |
Synopsis
Cricoid pressure during bag mask ventilation and during intubation has historically been utilized to minimize the risk of regurgitation of gastric contents into the airway while potentially aiding in visualization of laryngeal structures.21,22 However, uniform application of cricoid pressure may impede bag mask ventilation or obscure visualization of the airway during laryngoscopy.23-25 Cricoid pressure should be distinguished from external laryngeal manipulation where pressure is applied to the larger thyroid cartilage to facilitate airway visualization.26
Recommendation-Specific Supportive Text
1. A retrospective study from a large international pediatric ICU intubation registry showed that cricoid pressure during bag-mask ventilation before tracheal intubation was not associated with lower rates of regurgitation.26
2 and 3. A bronchoscopic study of 30 children revealed distortion of the airway with the application of cricoid pressure at forces below those recommended by usual intubation guidelines.27 Acknowledging anatomic differences in the airway from infancy through adulthood, relevant adult data was considered, given limited pediatric data for the use of cricoid pressure in pediatric patients. A systematic review of cricoid pressure during rapid sequence intubation in adult emergency department patients found insufficient evidence to conclude if cricoid pressure affected the rate of first pass intubation success or the incidence of complications such as aspiration.25 In a study of 60 adult patients undergoing elective anesthesia, cricoid pressure interfered with bag-mask ventilation in over 50% of patients.24
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. It may be reasonable to use atropine as a premedication to prevent bradycardia in infants and children during emergency intubation. |
| 2b | C-LD | 1. When atropine is used as a premedication for emergency intubation of infants and children, a dose of 0.02 mg/kg with no minimum dose, may be considered. |
Synopsis
Pediatric emergency intubation is a lifesaving, high-risk procedure that can be complicated by hypoxemia, bradycardia, hypotension, and cardiac arrest. Peri-intubation bradycardia may occur in response to (1) the underlying disease process that led to respiratory failure, (2) hypoxemia, (3) medications given for rapid sequence intubation, (4) the transition from negative to positive pressure ventilation, and (5) vagus nerve stimulation during laryngoscopy and endotracheal intubation, particularly in infants.28,29 Previous guidelines have discouraged the routine administration of atropine before intubation, but state that atropine pretreatment may be reasonable to prevent vagally mediated bradycardia in select populations.30 Observational studies demonstrate an association between atropine pretreatment and decreased bradycardia, higher heart rates without ventricular arrhythmias, and increased ICU survival.31,32 However, there is little evidence to support the use of atropine as a pretreatment for the prevention of peri-intubation cardiopulmonary compromise or cardiac arrest.
Recommendation-Specific Supportive Text
- Two prospective, observational studies conducted between 2007 and 2009 in patients less than 8 years of age intubated in the PICU or by critical care transport teams showed associations between atropine pretreatment and (1) decreased ICU mortality after propensity score adjustment and (2) significant acceleration in heart rate without provoking arrhythmias and a decrease in bradycardia.31,32 Conversely, 4 retrospective, observational studies did not demonstrate an association between atropine use and occurrence of bradycardia during the peri-intubation period.33-36
- Historically, a minimum dose of atropine (0.1 mg) was recommended to prevent paradoxical bradycardia based on a 1971 study of 79 patients undergoing elective surgery, of which 5 participants were between 6 weeks and 3 years of age. These infants and children had a small and statistically insignificant decrease in heart rate after receiving doses of atropine ranging from 0.0018 to 0.0036 mg/kg, approximately 10% to 20% of the recommended dose for atropine pretreatment for intubation.37 In 2015, in a prospective, observational study of 60 infants less than 15 kg undergoing elective surgery all of whom received less than 0.1 mg of atropine before intubation, no patient experienced paradoxical bradycardia or arrhythmias.38
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In all settings, for infants and children with a perfusing rhythm, exhaled CO2 detection (colorimetric detector or capnography) should be used for confirmation of ETT placement. |
| 2a | C-LD | 2. In infants and children with a perfusing rhythm and ETT in place, it is beneficial to monitor exhaled CO2 (colorimetric detector or capnography) during out-of-hospital and intra-hospital transport. |
Synopsis
Confirmation of ETT placement in patients with a perfusing rhythm is not reliably achieved by auscultation of breath sounds, mist in the tube, or chest rise. Either colorimetric exhaled CO2 detection or capnography is a more reliable method to assess initial ETT placement. In patients with decreased pulmonary blood flow from low cardiac output or cardiac arrest, exhaled CO2 may not be reliable.
Recommendation-Specific Supportive Text
- Although there are no randomized controlled trials linking use of exhaled CO2 detection with clinical outcomes, the Fourth National Audit Project of the Royal College of Anesthetists and the Difficult Airway Society concluded that the failure to use capnography contributed to adverse events, including death and persistent neurological injury, in a study of adults and children in the ICU and emergency department settings.39 One small, randomized study showed that capnography was faster than clinical assessment in premature newborns intubated in the delivery room.40 There has been no difference in patient outcomes in pediatric studies comparing qualitative (colorimetric) and quantitative (capnography or numeric display) exhaled CO2 detectors in the delivery room, ICU, or emergency department.41-43
- Adult literature suggests monitoring and correctly interpreting capnography in intubated patients may prevent adverse events.39,44,45 This has been demonstrated in pediatric simulation studies, in which capnography improved health care professional recognition and response to ETT dislodgement.46,47
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In infants and children with bradycardia and cardiopulmonary compromise due to increased vagal tone or atrioventricular conduction block not due to factors such as hypoxia, atropine is recommended. |
| 1 | C-EO | 2. In infants and children with heart rate less than 60 beats/min and cardiopulmonary compromise despite effective ventilation with oxygen, start CPR. |
| 1 | C-EO | 3. In infants and children with bradycardia and cardiopulmonary compromise that persists after correction of other factors (eg, hypoxia), give epinephrine IV/IO. |
| 2b | C-EO | 4. Emergency transcutaneous pacing may be considered for infants and children with bradycardia and cardiopulmonary compromise due to complete heart block or sinus node dysfunction unresponsive to CPR. |
Synopsis
Bradycardia associated with cardiopulmonary compromise, even with a palpable pulse, may be a harbinger of impending pulseless cardiac arrest. As such, bradycardia with a heart rate of less than 60 beats per minute in infants and children should prompt an evaluation for cardiopulmonary compromise, including acutely altered mental status, hypotension or other signs of shock. Correctable factors that contribute to bradycardia include hypoxia, hypotension, hypoglycemia, hypothermia, acidosis, and toxic ingestions.
Recommendation-Specific Supportive Text
- Atropine increases heart rate in both children and adults.1-4 As atropine is a vagolytic agent, use is limited to bradycardia with cardiopulmonary compromise due to increased vagal tone or atrioventricular conduction block, but not due to other factors such as hypoxia.
- Retrospective studies have shown that children who received CPR for bradycardia with cardiopulmonary compromise have better outcomes than children who receive CPR for pulseless cardiac arrest.5-8 No studies compared outcomes between children with bradycardia and cardiopulmonary compromise who did or did not receive CPR, though 1 study reported 8 patients who received drug therapy without CPR, and all survived to hospital discharge.9
- A retrospective, time-dependent propensity–score matched study of pediatric patients with bradycardia and cardiopulmonary compromise found that patients who received epinephrine had worse outcomes than patients who did not receive epinephrine.10 A subsequent retrospective study did not show differences in outcomes for patients who did or did not receive “early” epinephrine within the first 2 minutes of CPR for bradycardia and cardiopulmonary compromise.11 Due to limitations of these studies, further research on the impact of epinephrine on patients with bradycardia and cardiopulmonary compromise is required.
- There are limited data about transcutaneous pacing for refractory bradycardia in children with or without congenital/acquired heart disease.12-14 In patients with complete heart block or sinus node dysfunction who have not responded to oxygenation, ventilation, medications, or CPR, emergency transcutaneous pacing may be considered.
Figure 6 shows the algorithm for pediatric bradycardia with a pulse.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In infants and children with SVT without cardiopulmonary compromise, it is useful to attempt vagal stimulation first. |
| 1 | C-LD | 2. If IV/IO access is readily available, adenosine is recommended for the treatment of SVT in infants and children. |
| 1 | C-EO | 3. For infants and children without cardiopulmonary compromise whose SVT is unresponsive to vagal maneuvers and IV adenosine, expert consultation is recommended. |
| 2a | C-LD | 4. In infants and children with SVT and cardiopulmonary compromise, it is reasonable to perform electrical synchronized cardioversion starting with a dose of 0.5 to 1 J/kg. If unsuccessful, increase the dose to 2 J/kg. |
| 2b | C-LD | 5. For infants and children with SVT and cardiopulmonary compromise unresponsive to vagal maneuvers, adenosine, and electrical synchronized cardioversion and for whom expert consultation is not available, it may be reasonable to consider either IV procainamide, amiodarone, or sotalol. |
Synopsis
Regular, narrow-complex tachyarrhythmias (QRS duration 0.09 second or less) are most commonly caused by re-entrant circuits, although other mechanisms such as ectopic atrial tachycardia or junctional tachycardia may occur. Regular, wide-complex tachyarrhythmias (WCT; QRS greater than 0.09 second) can have multiple mechanisms, including SVT with aberrant conduction, antidromic SVT or VT. SVT with aberrant conduction is the most common cause of WCT in infants and children.1
The hemodynamic impact of SVT in the pediatric patient can be variable, with signs of cardiopulmonary compromise such as altered mental status, signs of shock or hypotension occurring in a minority of patients. In infants and children without cardiopulmonary compromise, re-entrant SVT can often be terminated with vagal maneuvers.2-5 Adenosine remains the preferred medication initially unresponsive to vagal maneuvers.3,6-14 For patients with hemodynamically stable SVT that recurs after initial successful treatment, expert consultation is important to diagnose etiology and customize treatment. The use of other IV antiarrhythmics including procainamide, amiodarone and sotalol have been studied with varying levels of success.15-20 When cardioversion is provided, the administration of sedation before synchronized cardioversion can minimize the pain associated with the shock. However, extreme caution is warranted in the selection and dosage of sedatives in a patient with cardiopulmonary compromise.
Recommendation-Specific Supportive Text
- Vagal maneuvers are noninvasive, have few adverse effects, and effectively terminate SVT in many cases; exact success rates for each type of maneuver (ie, ice water to face, postural modification) are unknown.3 Although improved success rates have been reported with a postural modification to the standard Valsalva maneuver in adults,2 published pediatric experience with this technique is very limited. Upside-down positioning may be an additional form of a vagal maneuver that is effective in children.4
- IV adenosine remains generally effective for terminating re-entrant SVT within the first 2 doses. In retrospective observational studies on the management of tachyarrhythmias, none directly compared adenosine to other drugs.6,7,9-14 A Cochrane review that included mostly adult studies and 1 pediatric study showed that either adenosine or calcium channel blockers can be used for quick termination of SVT.8 Given the fast onset of action, short half-life and favorable side effect profile, adenosine is preferred for infants and children.21
- For infants and children with SVT without cardiopulmonary compromise that is refractory to vagal maneuvers or adenosine, expert consultation can guide the choice of alternative second-line agents. The risk for proarrhythmic and life-threatening hemodynamic collapse increases with the administration of multiple antiarrhythmic agents. Multiple medications have been used as second-line agents for the management of adenosine-refractory SVT, including IV verapamil, β-blockers, amiodarone, procainamide, and sotalol.3,6,7,10,11,15,16,18-20,22,23 Few comparative studies exist.
- Direct current transcutaneous synchronized cardioversion remains the treatment of choice for infants and children with SVT and cardiopulmonary compromise (characterized by altered mental status, signs of shock, or hypotension). However, these cases are uncommon, and there are few studies that report outcomes from cardioversion of SVT.7,13,24 The administration of sedation before synchronized cardioversion can minimize the pain associated with the shock. However, extreme caution is warranted in the selection and dosage of sedatives in a patient with cardiopulmonary compromise.
- Procainamide and amiodarone are moderately effective treatments for adenosine-resistant SVT.16 Procainamide may be slightly more efficacious; adverse effects are frequent with both therapies. IV sotalol was approved for the treatment of SVT in 2009. Since its approval, several single-center and 1 multicenter registry have shown that IV sotalol is effective in acute conversion of SVT, with a 60% to 100% termination rate of SVT and atrial tachyarrhythmias.15,17,18-20 In these studies, IV sotalol was administered under the guidance of pediatric electrophysiologists in acute care settings and no significant adverse events were reported.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In infants and children with wide-complex tachycardia without cardiopulmonary compromise, expert consultation is recommended prior to administration of antiarrhythmic agents. |
| 2a | C-EO | 2. In infants and children with wide-complex tachycardia with regular and monomorphic QRS without cardiopulmonary compromise, adenosine administration can be useful in conjunction with expert consultation. |
| 2a | C-EO | 3. In infants and children with wide-complex tachycardia and cardiopulmonary compromise, it is reasonable to perform electrical synchronized cardioversion starting with a dose of 0.5–1 J/kg. If unsuccessful, increase the dose to 2 J/kg. |
Synopsis
The occurrence of WCT (QRS duration greater than 0.9 second) with a pulse is rare in children and may originate from either the ventricles or the atria.25 SVT with aberrant conduction is the most common cause of WCT in infants and children; however, other etiologies can include antidromic SVT or VT.1 For patients with WCT and no cardiopulmonary compromise, expert consultation is important to diagnose etiology and customize treatment. The administration of sedation before synchronized cardioversion can minimize the pain associated with the shock. However, extreme caution is warranted in the selection and dosage of sedatives in a patient with cardiopulmonary compromise.
Recommendation-Specific Supportive Text
- Both pediatric and adult studies have identified potential populations at risk of proarrhythmic complications from antiarrhythmic therapies, including patients with underlying cardiomyopathies, long-QT syndrome, Brugada syndrome, and Wolff-Parkinson-White syndrome.26-30 Given this potential risk, expert consultation can help provide a tailored antiarrhythmic choice to minimize the chances of arrhythmia.
- Given that the most common cause of WCT in infants and children is SVT with aberrancy,1 the use of adenosine can be either therapeutic or diagnostic. If the cause of the WCT is SVT with aberrancy, administration of adenosine is likely to terminate the arrhythmia. If the cause of the WCT is VT, the administration of adenosine can be diagnostic if it demonstrates ventriculo-atrial dissociation. Adenosine should only be administered in WCT if the tachycardia is monomorphic and regular. If WCT is irregular, it can represent pre-excited atrial fibrillation, and the administration of adenosine can lead to VF. When possible, the administration of adenosine should be done with a running rhythm strip or multilead electrocardiogram to help with interpretation of the adenosine response.1,31
- Cardiopulmonary compromise is a key factor in determining the use of electrical therapy over primary pharmacologic management in children with WCT and a pulse. In patients with cardiopulmonary compromise, electrical direct current synchronized cardioversion should be provided urgently, regardless of suspected atrial or ventricular origin. There is insufficient evidence describing the incidence of WCT without cardiopulmonary compromise, and there is no support for or against the use of specific antiarrhythmic drugs in the management of children with wide-complex tachycardia with a pulse.
Figure 7 shows the algorithm for pediatric tachyarrhythmia with a pulse.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Given the high risk of cardiac arrest in infants and children with myocarditis who demonstrate arrhythmias, heart block, ST-segment changes, or low cardiac output, early consideration of transfer to an ICU for monitoring and therapy is recommended. |
| 2a | B-NR | 2. For infants and children with myocarditis or cardiomyopathy with refractory low cardiac output, ECMO/mechanical cardiac support (MCS) can be beneficial to provide end-organ support and to prevent cardiac arrest. |
| 2a | B-NR | 3. In infants and children with myocarditis or cardiomyopathy experiencing cardiac arrest, early deployment of ECPR is reasonable. |
| 2a | C-LD | 4. For infants and children with myocarditis or cardiomyopathy and refractory low cardiac output, when feasible, early transfer to a center with ECMO/MCS capability is reasonable. |
Synopsis
Acute decompensated heart failure can result in end-organ compromise and rapid progression to cardiac arrest.1 Fulminant myocarditis and cardiomyopathy are common causes of acute decompensated heart failure in children.1-6 Outcomes can be optimized by early diagnosis and prompt intervention, including ICU monitoring and therapy. Heart block, ventricular arrythmias, requirement for invasive mechanical ventilation, or signs of organ failure in the patient with fulminant myocarditis are considered a prearrest state.2-5,7,8
Early consideration of transfer to a center capable of providing mechanical circulatory support (MCS) in the form of a ventricular assist device is important.3,4,9-11 Centers with more experience in the management of these patients may have better outcomes.3 Early ECMO cannulation of patients requiring invasive ventilation may be associated with survival.3,9 The use of ECMO and MCS have improved outcomes of patients with acute myocarditis, with a high possibility of partial or complete recovery of myocardial function.3-6,9,11-15
Recommendation-Specific Supportive Text
- Multiple retrospective studies have evaluated predictors of worse outcome in patients with acute decompensated heart failure with most studies in patients with fulminant myocarditis. There is an increased incidence of cardiac arrest and need for ECMO/MCS in this high-risk population.1-3,5,7,8,10,11 Incidence of cardiac arrest in fulminant myocarditis ranges from 25% to 75%.3,5,7,8,10 Signs of organ failure, a requirement for invasive mechanical ventilation, and even modest decreases in left ventricular fraction are associated with the need for ECMO/MCS.3-5,8,9,11 In 1 registry study, ventricular arrythmias that required treatment were strongly associated with mortality (OR, 8.47; 95% CI, 7.16–10.04; P less than 0.001).3
- The prognosis for patients with fulminant myocarditis who receive ECMO or MCS can be good and is generally better than for patients who require ECMO due to other causes, especially those with congenital heart disease.1,12 In 4 observational studies, transplant-free survival to hospital discharge in myocarditis patients without congenital heart disease requiring ECMO or MCS ranged from 72% to 80%.4,5,9,10
- In a cohort of 847 myocarditis patients, 60/847 (7.1%) had a cardiac arrest with 43/60 (72%) of these patients receiving ECPR. Among ECPR patients, 31/43 (72.1%) survived to hospital discharge compared to 11/17 (64.7%) of non-ECPR cardiac arrest patients.4 In 1 study, 95% of children with myocarditis who were placed on ECMO (n=15) or MCS (n=1) after cardiac arrest were alive 6 months later compared to 85% in nonmyocarditis patients.14
- In a retrospective study, shorter time to ECMO cannulation after intubation was associated with survival in patients with fulminant myocarditis (3 h in survivors, 6 h in nonsurvivors)9. A registry analysis from Japan noted improved outcomes if pediatric patients with myocarditis were managed in higher-volume centers3.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For infants and children with aortopulmonary shunt-dependent congenital heart disease with known or suspected shunt obstruction, early consideration for transfer to a center with advanced cardiac therapy capabilities (ie, cardiac catheterization, cardiac surgery, ECMO) is recommended if these services are not available. |
| 2a | C-LD | 2. In infants and children with single ventricle congenital heart disease following initial palliative procedures (eg, Stage I Norwood, Hybrid procedure, Blalock-Thomas-Taussig shunt), timely ECMO deployment can be useful to treat low cardiac output syndrome. |
| 2a | C-EO | 3. For infants and children with aortopulmonary shunt-dependent congenital heart disease with known or suspected shunt obstruction, it is reasonable to administer oxygen, vasoactive agents, and heparin while preparing for catheter-based or surgical intervention. |
| 2b | C-LD | 4. In infants and children with single ventricle congenital heart disease following initial palliative procedures (eg, Stage I Norwood, Hybrid procedure, Blalock-Thomas-Taussig shunt ) experiencing cardiac arrest, timely ECPR deployment may be a useful rescue strategy to achieve ROC. |
| 2b | C-LD | 5. In infants and children with single ventricle congenital heart disease following Stage II (bidirectional Glenn, hemi-Fontan) or Stage III (Fontan) palliation, timely ECMO deployment may be considered to treat low cardiac output syndrome. |
Synopsis
The complexity and variability in pediatric congenital heart disease (CHD) pose unique challenges during resuscitation. Children with single-ventricle CHD typically undergo 3 staged palliative operations. The first procedure, typically performed during the neonatal period, creates unobstructed systemic blood flow, an atrial communication to allow for atrial level mixing, and regulated pulmonary blood flow to prevent pulmonary overcirculation and to decrease the volume load on the systemic ventricle1,2 (Figure 8). During the second stage, a superior cavopulmonary anastomosis (ie, bidirectional Glenn/hemi-Fontan operation) is created to aid in the redistribution of systemic venous return directly to the pulmonary circulation (Figure 9). The Fontan procedure is the final palliation where inferior vena cava blood flow is baffled directly to the pulmonary circulation, thereby making the single (systemic) ventricle preload-dependent on passive flow across the pulmonary vascular bed (Figure 10).
Neonates and infants with single-ventricle physiology have an increased risk of cardiac arrest because of increased myocardial work from ventricular volume loading, imbalances in relative systemic (Qs) and pulmonary (Qp) blood flow, and potential shunt occlusion.1-3 Depending on the stage of repair, resuscitation may require control of pulmonary and systemic vascular resistance, administration of supplemental oxygen, intervention for shunt obstruction, or the use of ECMO.
Recommendation-Specific Supportive Text
- In aortopulmonary shunt-dependent CHD patients with suspected shunt obstruction, either cardiac catheterization or cardiothoracic surgery can be performed to restore patency of an obstructed shunt. Although observational single-center reports have not compared outcomes between procedural interventions (catheter-based or surgical) versus no procedural intervention for treatment of aortopulmonary shunt obstruction, lack of pulmonary blood flow can lead to severe cardiopulmonary compromise and cardiac arrest.1-4 ECMO can be deployed to rescue the shunt-dependent patient with cardiopulmonary compromise related to shunt obstruction before performing interventions to restore shunt patency.1-5
- For children with single ventricle CHD after initial surgical palliation (eg, Blalock-Thomas-Taussig shunt, Stage I Norwood) needing postoperative ECMO for management of low cardiac output state, reported survival rates range from 31% to 53%.3,5,7-9 In 1 small observational study of patients undergoing staged palliation, survival rates were statistically unchanged in patients who received elective ECMO (n=15) compared to ECPR (n=10) (55% versus 36%, P=0.17).7 In another study, neonates with hypoplastic left heart syndrome status post–Stage I palliation supported with ECMO had a survival rate of 31%, compared to 51% survival for neonates placed on ECMO for cardiac indications as reported to Extracorporeal Life Support Organization registry database.5 Though reported survival rates for ECPR in this cohort (32%–57%) after initial surgical palliation are similar to survival rates for elective ECMO (31%–53%), clinical practice standards advocate for earlier recognition of the postoperative low cardiac output state and deployment of elective ECMO before cardiac arrest, as this may be associated with improved long-term functional outcomes.5,7,10,11 There are no studies which directly compare children with single ventricle CHD after initial surgical palliation experiencing impending hemodynamic collapse receiving postoperative elective ECMO to cohorts not receiving ECMO under similar hemodynamic conditions.
- Medical treatment for acute aortopulmonary shunt obstruction in peri-arrest and cardiac arrest states can include administration of supplemental oxygen, vasoactive agents (eg, epinephrine, phenylephrine, norepinephrine) to maximize shunt perfusion pressure, and anticoagulation with heparin (50–100 units/kg bolus) to mitigate clot propagation.1,3,6
- In children with single ventricle CHD after initial surgical palliation (eg, Blalock-Thomas-Taussig shunt, Stage I Norwood) experiencing cardiac arrest, observational and registry studies identify rates of survival to hospital discharge ranging from 32% to 57%.3,5,7,9,11-16 One observational and 1 registry study of neonates with hypoplastic left heart syndrome undergoing ECPR after Stage I palliation noted survival rates of 32% to 36%, similar to the survival rate of patients undergoing elective ECMO (30%).5,16 There are no studies of single ventricle populations after initial stage palliation receiving ECPR for cardiac arrest that provide comparisons to conventional CPR. There are limited data on ECPR outcomes in children after Stage II and Stage III surgical palliation. Observational studies of ECMO support after Stage II palliation showed an overall survival rate of 41%, with sub analysis of ECPR in Stage II cohorts noting a survival rate of 44% to 45%17,18 and rates of neurological injury as high as 57% in Stage II ECPR survivors (Pediatric Overall Performance Category/PCPC score ≥3).18 One observational study of children receiving postoperative ECMO after Stage III surgical palliation identified a survival rate of 27% for the subgroup of Stage III cohorts receiving ECPR.19 Due to a lack of significant literature supporting ECPR use after Stage II and III surgical palliations,17-19 specific recommendations for ECPR in these cohorts are not provided.
- In a retrospective analysis of the Extracorporeal Life Support Organization database, among infants undergoing Stage II palliation receiving postoperative ECMO support, survival rates for those undergoing elective ECMO were similar to those patients undergoing ECPR (41% versus 45%, respectively), with a higher rate of neurological injury for the cohort who received ECMO versus ECPR (23% versus 0%).17 An Extracorporeal Life Support Organization database study evaluating children after Stage III palliation (Fontan) who received postoperative ECMO noted a survival rate of 35%.19 There are no studies which directly compare children with single ventricle CHD after Stage II or III surgical palliation experiencing impending hemodynamic collapse receiving postoperative elective ECMO to analogous cohorts not receiving ECMO under similar hemodynamic conditions.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For infants and children who are at risk for pulmonary hypertensive crises, provide careful respiratory management and monitoring to avoid hypoxia and acidosis. |
| 1 | C-EO | 2. For infants and children who are mechanically ventilated and at risk for pulmonary hypertensive crises, provide adequate analgesics, sedatives, and neuromuscular blocking agents. |
| 1 | C-EO | 3. For infants and children who are at risk for pulmonary hypertensive crises, avoid dehydration, fluid overload, and anemia. |
Synopsis
Pulmonary hypertensive crises are acute rapid increases in pulmonary artery pressure that can lead to systemic hypotension, myocardial ischemia, cardiac arrest, and death. Optimization of physiologic conditions and avoidance of potential triggers of pulmonary hypertensive crises can potentially avoid these events. Acidosis and hypoxemia are both potent pulmonary vasoconstrictors.1-4 Inadequate sedation or uncontrolled pain can lead to increased sympathetic activity, which raises pulmonary vascular resistance and can exacerbate pulmonary hypertension.1,5-7 Hypovolemia in children with pulmonary hypertension decreases preload and potentially compromises cardiac output, worsening the condition and increasing hypoxia risk. Conversely, fluid overload causes venous congestion and impairs heart function in the setting of right ventricular dysfunction.8 Anemia can be problematic in patients with pulmonary hypertension due to reduced oxygen-carrying capacity.8
Recommendation-Specific Supportive Text
- One review, 1 consensus statement, and 1 scientific statement highlight the importance of identifying and treating environmental factors that contribute to pediatric pulmonary hypertensive crises, such as hypoxia and acidosis, especially in high-risk populations with preexisting idiopathic pulmonary arterial hypertension or with congenital heart disease and pulmonary arterial hypertension.7,9,10 Two physiologic reviews, 1 randomized clinical trial, and 2 retrospective observational studies demonstrated that hypercarbia, hypoxemia, acidosis, atelectasis, and ventilation-perfusion mismatch can lead to increases in pulmonary vascular resistance and, hence, elevation of pulmonary artery pressures in the immediate postoperative period.1-4,11
- Two observational studies in select high-risk postoperative cardiac patients found an attenuation in the stress response in those patients receiving fentanyl in the postoperative period.5,6 Three studies indicate that triggering factors, such as pain and anxiety, can precipitate pulmonary hypertension crises both during the perioperative period and outside of it, particularly in the presence of acute lung injury, infection, or noncardiac interventions. Managing and preventing these aggravating factors with sedatives and analgesics can help prevent recurrent, more severe, and prolonged pulmonary hypertension crises.7,10,12 Neuromuscular blockade can be used in addition to sedation and analgesia in critically ill, mechanically ventilated children with pulmonary hypertension.7,12
- Hypovolemia and hypervolemia can be deleterious to the cardiac function. Right ventricular decompensation may result from conditions leading to increases in cardiac demand or increases in ventricular afterload. Two consensus statements on the treatment of pediatric pulmonary hypertension emphasize the importance of managing triggering factors such as volume overload, dehydration, and anemia in critically ill children with acute pulmonary hypertension.8,10
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In infants and children with pulmonary hypertensive crises or acute right-sided heart failure secondary to increased pulmonary vascular resistance, inhaled nitric oxide or prostacyclin should be used as the initial pulmonary vasodilator therapy. |
| 2a | C-LD | 2. For the initial treatment of infants and children with pulmonary hypertensive crises, oxygen administration and induction of alkalosis through hyperventilation or alkali administration can be useful while pulmonary vasodilators are administered. |
| 2b | C-LD | 3. For infants and children with refractory pulmonary hypertension, including signs of low cardiac output or profound respiratory failure despite optimal medical therapy, ECMO may be considered. |
Synopsis
During a pulmonary hypertensive crisis, elevated pulmonary vascular resistance reduces pulmonary blood flow, impairing left-heart (or single ventricle) preload and ultimately leading to a fall in cardiac output. Hypoxia and hypercarbia can significantly increase pulmonary vascular resistance and trigger pulmonary hypertension crises.1,8,10 Supplemental oxygen helps maintain oxygen levels and lowers pulmonary artery pressures, as does inducing alkalosis. Pulmonary vasodilators, including inhaled nitric oxide, inhaled prostacyclin and IV prostacyclin analogs have been used to treat acute pulmonary hypertensive crises while IV and oral phosphodiesterase type-5 inhibitors, oral endothelin receptor antagonists and oral soluble guanylate cyclase stimulators are used as long-term therapies to lower pulmonary vascular resistance and prevent pulmonary hypertensive crises.13-16 ECMO may stabilize infants with severe pulmonary hypertension, low cardiac output, or severe respiratory failure when other treatments fail,12 or during high-risk cardiac interventions.17 Children with pulmonary hypertension who require ECMO have higher mortality rates compared to those without pulmonary hypertension.18,19
Recommendation-Specific Supportive Text
1 and 2. Treatment with inhaled nitric oxide reduces the frequency of pulmonary hypertensive crises and shortens time to extubation.20 In patients with atrioventricular septal defect repair and severe postoperative pulmonary hypertension, inhaled nitric oxide administration is associated with reduced mortality.15,21 Inhaled prostacyclin transiently produces pulmonary vasodilation and improves oxygenation, but the alkalinity of the drug can irritate airways, and precise dosing can be complicated by drug loss in the nebulization circuit.12,22 Two physiologic reviews and 1 randomized clinical trial demonstrated that hypercarbia, hypoxemia, acidosis, atelectasis, and ventilation-perfusion mismatch can lead to increases in pulmonary vascular resistance and elevation of pulmonary artery pressures in the immediate postoperative period after cardiac surgery.2-4 One prospective study on the right ventricular response to hypercarbia following cardiac surgery showed that hypercarbia significantly increased pulmonary vascular resistance by 54% and mean pulmonary arterial pressure by 34%.23 In addition, a prospective study in infants after cardiopulmonary bypass demonstrated that increasing the arterial pH by the administration of sodium bicarbonate resulted in significant reduction of pulmonary vascular resistance with both a decrease in mean pulmonary arterial pressure and a concomitant increase in cardiac index.24
3. Extracorporeal membrane oxygenation has been used in children with pulmonary vascular disease in refractory low cardiac output states and cardiac arrest.25,26 Although outcomes remain poor in certain populations,27 advances in technology of extracorporeal devices may allow for bridging to durable MCS or to transplantation.17,28 Although patients with pulmonary hypertension who require ECMO have a high mortality rate, provision of ECMO can be lifesaving.12,29,30
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-EO | 1. In infants and children with pulmonary hypertension experiencing cardiac arrest, it is unknown whether the addition of pulmonary hypertension–specific therapies improves CPR outcomes compared to standard CPR. |
Synopsis
Among children with in-hospital cardiac arrest, pulmonary hypertension is a common preexisting condition, and several observational studies have found an association between pulmonary hypertension and lower cardiac arrest survival rates.19,31-34 Despite inhaled nitric oxide showing efficacy in preclinical large animal models of cardiac arrest associated with pulmonary hypertension,35,36 studies on optimal management for children with pulmonary hypertension-associated cardiac arrest are scarce, and the efficacy of specific intra-arrest therapies is unknown.
Recommendation-Specific Supportive Text
- No intra-arrest therapies have been prospectively compared to standard resuscitation therapies for pulmonary hypertension-associated cardiac arrest. One observational study reported that 55% of children with pulmonary hypertension and in-hospital cardiac arrest were receiving inhaled nitric oxide at the time of arrest, but did not identify associations with survival.32 No studies have prospectively investigated pulmonary vasodilator therapy during pediatric cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In infants and children with traumatic cardiac arrest, evaluation and treatment for potentially reversible causes such as hemorrhage, tension pneumothorax, and pericardial tamponade are recommended. |
| 2b | C-LD | 2. In infants and children with traumatic cardiac arrest secondary to penetrating thoracic injury with a short transport time, it may be reasonable to perform resuscitative thoracotomy. |
Synopsis
Unintentional injuries are the most common cause of death among children and adolescents.1 Although many organizations have established trauma care guidelines,2-4 the management of traumatic cardiac arrest is often inconsistent. Cardiac arrest due to major blunt or penetrating injury in children has a very high mortality rate.5-8 Suspect thoracic injury in all thoraco-abdominal trauma because tension pneumothorax, hemothorax, pulmonary contusion, or pericardial tamponade may impair hemodynamics, oxygenation, and ventilation.
This topic was last reviewed in the 2020 AHA Guidelines for CPR and ECC. These recommendations have not been updated for this edition of the Guidelines.9
Recommendation-Specific Supportive Text
- Early correction of reversible causes by reducing delays in the delivery of trauma-specific interventions may increase survival following penetrating traumatic cardiac arrest.10,11 Guidelines for cardiac arrest due to trauma recommend opening the airway, relieving tension pneumothorax hemorrhage control, restoring circulating blood volume, and relieving tension pneumothorax. Perform these measures simultaneously with conventional resuscitation.
- Systematic reviews,12-15 multicenter retrospective studies,16,17 and single-center retrospective studies18 recommend emergent thoracotomy for pediatric patients who present pulseless after penetrating thoracic injury. However, there is no evidence to support emergent thoracotomy for infants and children with blunt injury presenting in cardiac arrest.13,19
Critical Knowledge Gaps and Ongoing Research
During the literature review process, we identified several critical knowledge gaps related to PALS. These topics are either current areas of ongoing research or lack significant pediatric evidence to support evidence-based recommendations.
The causes of pediatric cardiac arrest are very different from cardiac arrest in adults, and pediatric studies are critically needed. As often occurs in the development of pediatric medical guidelines, some recommendations are extrapolated from adult data. However, infants and children are distinct patient populations and dedicated pediatric resuscitation research is a priority given the more than 20 000 cardiac arrests that occur in infants and children in the United States each year. These critical knowledge gaps are identified areas that, when addressed, will be the foundational future knowledge to advance how we care for patients.
Critical knowledge gaps are summarized in Table 2.
Table 2. Critical Knowledge Gaps Due to Insufficient Pediatric Data
|
Topic |
Knowledge gap |
|
Guidelines development |
What is the appropriate age and setting to transition from (1) neonatal resuscitation protocols to pediatric resuscitation protocols and (2) from pediatric resuscitation protocols to adult resuscitation protocols? |
|
Drug administration |
What is the optimal method (ie, age, weight, or physiology) to estimate body weight for medication administration? |
|
What is the optimal method to transition from weight-based dosing to fixed-dose medication administration during cardiac arrest in adolescents or children with high BMI? |
|
|
With what frequency should subsequent doses of epinephrine be administered? |
|
|
Should epinephrine dosing strategies be individualized to patients based on hemodynamic response, blood pressure targets, or both? |
|
|
With what frequency should epinephrine be administered in infants and children during CPR who are undergoing ECMO cannulation? |
|
|
What are special circumstances in which sodium bicarbonate and calcium are beneficial to administer during pediatric cardiac arrest? Are there circumstances where these medications are harmful? |
|
|
CPR techniques |
Are alternative compression techniques (cough CPR, precordial thump, fist pacing, interposed abdominal compression CPR, mechanical CPR devices) equally or more effective than conventional CPR? |
|
What are the optimal chest compression techniques for patients immediately poststernotomy? |
|
| Is double sequential defibrillation, vector change, or stacked shocks more effective to achieve ROSC than traditional single defibrillation? When are these strategies appropriate in defibrillation sequences when single shocks have failed? | |
|
What is the optimal timing and dosing of initial defibrillation for VF/pVT or subsequent defibrillation for refractory VF/pVT? |
|
|
Airway management, ventilation, oxygenation during cardiac arrest |
Are there specific situations in which advanced airway placement is beneficial or harmful in OHCA? |
|
What is the appropriate timing of advanced airway placement in IHCA? |
|
|
What is the optimal Fio2 to administer during CPR? |
|
|
What is the optimal ventilation rate during CPR in patients with or without an advanced airway? Is it age dependent? |
|
|
What is the optimal PEEP, PIP, tidal volume to administer during CPR? |
|
|
For mechanically ventilated infants and children with IHCA, does manual ventilation versus remaining on the ventilator impact the efficacy of ventilation or outcomes? |
|
|
Is there an ETCO2 value that health care professionals should target during CPR? |
|
|
Monitoring during cardiac arrest |
What are the optimal blood pressure targets during CPR? Is it age dependent? |
|
Can transthoracic or transesophageal echocardiography diagnose reversible causes/contributors to cardiac arrest? |
|
|
Can transesophageal echocardiography during cardiac arrest guide and improve CPR quality? |
|
|
Can NIRS or other cerebral perfusion monitoring technologies improve CPR quality or outcomes when used during cardiac arrest? |
|
|
Should specific heart rate, blood pressure or other factors be used as the threshold for performing CPR in children with poor perfusion? |
|
|
Mechanical circulatory support (ECPR/VAD) |
What is the role of ECPR for patients with OHCA and IHCA due to noncardiac causes? |
|
What other patient/arrest factors should be considered in determining ECPR candidacy? |
|
|
Should the optimal dosing frequency of vasoactive medications be modified for ECPR? |
|
|
What approach to minimizing chest compression pauses during cannulation is optimal during ECPR? |
|
|
What is the role of ECPR deployment in patients with single ventricle heart disease at each stage of palliation (Stage I, II, III)? |
|
|
Should post–cardiac arrest care hemodynamic, oxygenation, ventilation targets be similar for ECPR patients when compared to conventional CPR patients? |
|
|
What are optimal CPR practices for patients with intra- or extracorporeal ventricular assist devices (continuous flow or pulsatile) experiencing low-flow alarm, absent pulse, hypotension, with or without evidence of VAD malfunction? |
|
|
Termination of resuscitation |
What clinical tools can be used to help in the decision to terminate pediatric IHCA and OHCA resuscitation? |
|
Family presence |
What is the long-term emotional and psychological impact on family members who witness resuscitation, including potential trauma, grief processing, or closure? |
|
What is the impact on health care professionals’ emotional well-being when families are present during resuscitation? |
|
|
How do cultural, religious, or individual family beliefs influence the perception of FPDR and how should we tailor practices to respect these differences? |
|
|
Post–cardiac arrest care, neuroprognostication |
What are the blood pressure targets during post–cardiac arrest period and for what time period post–cardiac arrest should they serve as hemodynamic targets? |
|
Should seizure prophylaxis be administered post-cardiac arrest? |
|
|
Does the treatment of post–cardiac arrest convulsive and nonconvulsive seizure improve outcomes? |
|
| What is the optimal temperature and duration of TTM for post–cardiac arrest care? | |
|
Does TTM benefit subpopulations of cardiac arrest patients? |
|
|
What are the reliable methods and optimal timing for post–cardiac arrest prognostication? |
|
|
What are the modalities and timing of physical, cognitive, and psychological assessments that should be provided in the year following cardiac arrest? |
|
|
Which modalities and their respective timing should be used to predict good and poor neurological outcomes after cardiac arrest? |
|
|
Arrhythmia management |
What are the most effective and safe medications for adenosine-refractory SVT? |
|
Specific disease etiologies presenting in cardiac arrest – myocarditis/cardiomyopathy |
Are there specific modifications to the cardiac arrest algorithm that should be used when treating a patient with myocarditis/cardiomyopathy and cardiac arrest? |
|
Specific disease etiologies presenting in cardiac arrest – pulmonary hypertension |
What intra-arrest therapies are effective to treat pulmonary hypertension-associated cardiac arrest compared to standard resuscitation therapies? |
|
What is the optimal approach to ventilation during the resuscitation of infants and children with pulmonary hypertension? |
The American Heart Association requests that this document be cited as follows: Lasa JJ, Dhillon GS, Duff JP, Hayes J, Kamath-Rayne BD, Levy A, Mahgoub M, Morgan RW, McCormick T, Roberts JS, Ross CE, Schexnayder SM, Sweberg T, Valdes SO, Topjian AA. Part 8: pediatric advanced life support: 2025 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2025; 152(suppl 2):S479–S537. doi: 10.1161/CIR.0000000000001368
This article has been copublished in Pediatrics.
- Javier J. Lasa, MD, Co-Chair
- Gurpreet S. Dhillon, MD
- Jonathan P. Duff, MD, Med
- Jennifer Hayes, RN, MSN
- Beena D. Kamath-Rayne, MD, MPH
- Arielle Levy, MD, Med
- Melissa Mahgoub, PhD
- Ryan W. Morgan, MD, MTR
- Taylor McCormick, MD
- Joan S. Roberts, MD
- Catherine E. Ross, MD
- Stephen M. Schexnayder, MD
- Todd Sweberg, MD, MBA
- Santiago O. Valdés, MD
- Alexis A. Topjian, MD, MSCE, Co-Chair
Contributor: Rebecca N. Ichord, MD
Open table in a new window.
Open table in a new window.
 Login
Login