Part 6: Pediatric Basic Life Support
Abstract
Codeveloped by the American Heart Association and the American Academy of Pediatrics, this publication presents the 2025 guidelines for basic life support during cardiopulmonary resuscitation and emergency cardiovascular care of the pediatric patient, excluding the newborn infant, and represents the first comprehensive update of treatment recommendations since 2020. Incorporating the results of structured evidence reviews from the International Liaison Committee on Resuscitation, these guidelines are for lay rescuers and health care professionals with recommendations designed to improve survival from sudden cardiac arrest and acute life-threatening cardiopulmonary problems. Existing guidelines remain relevant unless specifically updated in this publication. Topics reviewed include the initiation of cardiopulmonary resuscitation; pulse check; components of high-quality cardiopulmonary resuscitation; chest compression technique; support surfaces for cardiopulmonary resuscitation; opening the airway; coordination of shock and cardiopulmonary resuscitation; types of defibrillators or automated external defibrillators; defibrillator paddle or pad size, type, position; treatment of inadequate breathing with a pulse; and foreign body airway obstruction. Key topics that are new, are substantially revised, or have significant new literature include the elimination of 2-finger chest compressions in infants due to ineffectiveness of achieving proper depth with a recommendation of 1-hand or 2 thumb–encircling hands technique; the immediate application and use of an automated external defibrillator with a pediatric attenuator if available for cardiac arrest; and in infants with severe foreign body airway obstruction repeated cycles of 5 back blows alternating with 5 chest thrusts (no abdominal thrusts), and in children with severe foreign body airway obstruction repeated cycles of 5 back blows alternating with 5 abdominal thrusts.
Introduction
- Respiratory conditions remain the major cause of cardiac arrest in infants and children and, as such, appropriate interventions to support ventilation and oxygenation should be initiated quickly.
- For out-of-hospital cardiac arrest (OHCA) in infants and children, providing breaths in addition to chest compressions improves survival; thus, lay rescuers are encouraged to provide breaths if able and willing.
- A respiratory rate of 20 to 30 breaths per minute is recommended for infants and children who are (a) receiving cardiopulmonary resuscitation (CPR) with an advanced airway in place or (b) receiving breaths and have a pulse.
- For infants with severe foreign-body airway obstruction (FBAO), repeated cycles of 5 back blows alternating with 5 chest thrusts is recommended. Abdominal thrusts are not recommended in infants.
- For children with severe FBAO, repeated cycles of 5 back blows alternating with 5 abdominal thrusts is recommended.
- Immediate recognition of cardiac arrest is vital to improving outcomes. For infants and children who are unresponsive with abnormal breathing including gasping, rescuers should activate emergency medical services and initiate high-quality CPR beginning with chest compressions.
- High-quality CPR is the foundation of resuscitation. The key components of high-quality CPR include providing adequate chest compression rate and depth, minimizing interruptions in CPR, allowing complete chest recoil between compressions, and avoiding excessive ventilation.
- For infants, the recommended compression techniques include using either the 1-hand technique or the 2 thumb–encircling hands technique. If the rescuer cannot physically encircle the chest, it is recommended to compress the chest with the heel-of-1-hand technique. The use of 2 fingers along the sternum was eliminated due to ineffectiveness in achieving proper depth.
- For infants and children in cardiac arrest, an automated external defibrillator (AED) should be attached as soon as possible using a pediatric attenuator and pediatric pads if available.
- Prompt defibrillation for ventricular fibrillation and pulseless ventricular tachycardia (pVT) is critical, with minimization of peri-shock pauses.
Preamble
More than 20000 infants and children have an in-hospital cardiac arrest (IHCA) per year in the United States.1-5 In 2015, emergency medical service–documented OHCA occurred in >7000 infants and children.4 Pediatric OHCA patients’ survival to hospital discharge varies by age, with survival rates of 17.3% in adolescents, 14.7% in children, and 6.6% in infants.6 Since 2000, pediatric IHCA has had an overall increase in survival to hospital discharge improving from 18.9% to 44.2% in 2022.5 Neurologic outcomes remain difficult to assess across the pediatric age spectrum, with variability in reporting metrics and time to follow-up across studies of both OHCA and IHCA. Favorable neurological outcome has been reported in up to 47% of survivors to discharge.7 Despite increases in survival from IHCA, there is more to be done to improve both survival and neurological outcomes.8
These guidelines have recommendations for pediatric basic life support (BLS)—excluding the newborn infant—and are based on the best available resuscitation science. The Chain of Survival (see Major Concepts section) including recovery from cardiac arrest, requires coordinated efforts from medical professionals in a variety of disciplines and, in the case of OHCA, from lay responders, emergency dispatchers, and first responders. In addition, specific recommendations for pediatric advanced life support are provided in “Part 8: Pediatric Advanced Life Support”9; recommendations for resuscitation training are provided in “Part 12: Resuscitation Education Science”10; recommendations about systems of care are provided in “Part 4: Systems of Care”11; recommendations for special circumstances are provided in “Part 10: Adult and Pediatric Special Circumstances of Resuscitation”12; and ethical considerations related to pediatric resuscitation are discussed in “Part 3: Ethics.”13
Scope of the Guidelines
These guidelines are intended to be a resource for lay responders and health care professionals to identify and treat infants and children in the prearrest, intra-arrest, and post-arrest states. These apply to infants and children in multiple settings: the community, prehospital, and the hospital environment. Prearrest, intra-arrest, and postarrest topics are reviewed, including cardiac arrest in special circumstances such as severe FBAO.
For pediatric BLS, guidelines apply as follows:
- Infant guidelines apply to infants younger than approximately 1 year of age (excluding newborn infants).
- Child guidelines apply to children approximately 1 year of age until puberty. For teaching purposes, puberty is defined as breast development in females and the presence of axillary hair in males.
- For those with signs of puberty and beyond, adult BLS guidelines should be followed.
Neither pediatric advanced nor basic life support guidelines address the resuscitation of newborn infants, who are transitioning from a fluid-filled to an air-filled environment. Resuscitation of the newborn infant is addressed in “Part 5: Neonatal Resuscitation”14. Although pediatric basic and advanced life support guidelines may be applied to newborn infants <28 days old based on pathophysiology and institutional practice, neonatal guidelines should be followed at birth to address unique aspects of transitional physiology.15 Infancy is considered the first year of life.
Organization of the Pediatric Writing Committee
The Pediatric BLS Writing Group consisted of pediatric clinicians with representation from the American Heart Association (AHA) and the American Academy of Pediatrics (AAP) including intensivists, cardiac intensivists, emergency medicine physicians, respiratory therapists, nurses, and nurse practitioners. A call for candidates was distributed to the AHA Emergency Cardiovascular Care Committee and AAP subject matter experts, and volunteers with recognized expertise in pediatric resuscitation were nominated by the writing group cochairs. Writing group members were selected by the AHA Emergency Cardiovascular Care Science Subcommittee and AAP Executive Committee and then approved by the AHA Manuscript Oversight Committee. The AHA and AAP have rigorous conflict of interest policies and procedures to minimize the risk of bias or improper influence during development of the guidelines.16 Prior to appointment, writing group members and peer reviewers disclosed all commercial relationships and other potential (including intellectual) conflicts. Writing group members whose research led to changes in guidelines were required to declare those conflicts during discussions and abstain from voting on those specific recommendations. This process is described more fully in “Part 2: Evidence Evaluation and Guidelines Development.”17 Comprehensive disclosure information for writing group members and peer reviewers is listed in Appendixes 1(link opens in new window) and 2.
Methodology and Evidence Review
These pediatric guidelines are based on the extensive evidence evaluation performed in conjunction with the International Liaison Committee on Resuscitation and affiliated
International Liaison Committee on Resuscitation member councils. Three types of evidence reviews (systematic reviews, scoping reviews, and evidence updates) were used in the 2025 process.18,19 This process is described more fully in “Part 2: Evidence Evaluation and Guidelines Development.”17
Class of Recommendation and Level of Evidence
The writing group reviewed all relevant and current AHA Guidelines for CPR and Emergency Cardiovascular Care and all relevant International Liaison Committee on Resuscitation Consensus on CPR and Emergency Cardiovascular Care Science With Treatment Recommendations from 2020, 2022, 2023, and 2024.20-23 Evidence and recommendations were reviewed to determine if current guidelines should be reaffirmed, revised, or retired, or if new recommendations were needed. The writing group then drafted, reviewed, and approved recommendations, assigning a Class of Recommendation (COR; ie, strength) and Level of Evidence (LOE; ie, quality, certainty) to each. Criteria for each COR and LOE are described in Table 1.
Open table in a new window.
Guideline Structure
The 2025 guidelines are organized in discrete modules of information on specific topics or management issues.24 Each modular knowledge chunk includes a table of recommendations using standard AHA nomenclature of COR and LOE. Recommendations are presented in order of COR: most potential benefit (Class 1), followed by lesser certainty of benefit (Class 2), and finally potential for harm or no benefit (Class 3). Following the COR, recommendations are ordered by the certainty of supporting LOE: Level A (high-quality randomized controlled trials) to Level C-EO (expert opinion). This order does not reflect the order in which care should be provided.
In addition to the table of recommendations, the knowledge chunk includes a brief synopsis to put the recommendations into context with important background information and overarching management or treatment concepts. Recommendation-specific supportive text clarifies the rationale and key study data supporting the recommendations. When appropriate, illustrations are included. Hyperlinked references are provided to facilitate quick access and review.
Document Review and Approval
The guideline was submitted for blinded peer review to 8 subject matter experts nominated by the AHA and AAP. Before appointment, all peer reviewers were required to disclose relationships with industry and any other conflicts of interest, and all disclosures were reviewed by AHA staff. The guideline was also reviewed and approved for publication by the AHA Science Advisory and Coordinating Committee, the AHA Executive Committee, and the AAP Board of Directors. Comprehensive disclosure information for peer reviewers is listed in Appendixes 1(link opens in new window) and 2(link opens in new window). These recommendations supersede the last full set of AHA recommendations for pediatric advanced life support, made in 2020.25 The writing group consisting of AHA and AAP representatives voted on and approved all guideline recommendations.
Major Concepts
The epidemiology, pathophysiology, and common etiologies of pediatric cardiac arrest are distinct from adult and neonatal cardiac arrest. Cardiac arrest in infants and children does not usually result from a primary cardiac cause; rather, it is the result of progressive respiratory failure or shock. In these patients, cardiac arrest is preceded by a variable period of deterioration, which eventually results in cardiopulmonary failure, bradycardia, and cardiac arrest. In children with congenital heart disease, cardiac arrest is often due to a primary cardiac cause, although the etiology is distinct from that in adults.
Outcomes for pediatric IHCA have improved over the past 20 years, in part because of early recognition, high-quality CPR, post-arrest care, and extracorporeal CPR.1-3 In a recent analysis of the AHA Get With the Guidelines-Resuscitation Registry, a large multicenter, hospital-based cardiac arrest registry, pediatric IHCA survival to hospital discharge was 19% in 2000 and 44% in 2022. Survival has improved for pediatric events requiring CPR in the United States, with a 19% absolute increase in survival for in-hospital pulseless cardiac arrests and a 9% absolute increase in survival for nonpulseless events (eg, bradycardia with hemodynamic compromise) between 2000 and 2018. However, survival from pulseless cardiac arrests has reached a plateau following 2010.2 New directions of research and therapy may be required to improve cardiac arrest survival. More IHCA events now occur in an intensive care unit setting, suggesting that patients at risk for cardiac arrest are being identified sooner and transferred to a higher level of care.4,5
Survival rates from OHCA remain less encouraging. Most pediatric OHCA are respiratory/asphyxial events leading to asystole or PEA and thus are nonshockable rhythms. While AEDs may be more prevalent in schools and sporting events over recent years, AEDs can deliver shocks only if a shockable rhythm (VF/pVT) is detected. AEDs save lives in the minority of pediatric arrests with shockable rhythms, but because those cases are rare, widespread AED deployment has not yet resulted in large overall improvements in pediatric OHCA survival at the population level.6-8 In a recent analysis of the Cardiac Arrest Registry to Enhance Survival from 2022, a multicenter OHCA registry, annual survival to hospital discharge of pediatric OHCA ranged from 6.6% to 17.3% depending on region and patient age, demonstrating gains for some populations over the last decade.9 There was no significant change in these rates over time, consistent with other national registries.10,11 In the Resuscitation Outcomes Consortium Cardiac Epidemiological Registry, survival after OHCA was higher in regions with more arrests that were witnessed by emergency medical services and with higher lay responder CPR rates, stressing the importance of early recognition and treatment of these patients.4
As survival rates from pediatric cardiac arrest increase, there has been a shift with more focus on neurodevelopmental, physical, and emotional outcomes of survivors. Recent studies demonstrate that one quarter of patients with favorable outcomes have global cognitive impairment and that 85% of older children who were reported to have favorable outcomes have selective neuropsychological deficits.12
The Pediatric Chain of Survival
Historically, cardiac arrest care has focused on the management of the cardiac arrest itself, highlighting high-quality CPR, early defibrillation, and effective teamwork. However, there are aspects of pre-arrest and post-arrest care that are critical to improve outcomes. As pediatric cardiac arrest survival rates have plateaued for pulseless cardiac arrest, the prevention of cardiac arrest becomes even more important. In the out-of-hospital environment, this includes safety initiatives (eg, bike helmet laws), sudden infant death syndrome prevention, lay rescuer CPR training, early recognition of the critically ill infant or child by the caregiver, and timely access to emergency care. When OHCA occurs, early lay responder CPR is critical in improving outcomes, as well as the availability of AEDs for shockable rhythms. In the in-hospital environment, cardiac arrest prevention focuses on the early recognition and treatment of patients at high risk, such as children undergoing cardiac surgery or children with acute fulminant myocarditis, acute decompensated heart failure, or pulmonary hypertension. Following resuscitation from cardiac arrest, management of the post–cardiac arrest syndrome (which may include brain dysfunction, myocardial dysfunction with low cardiac output, and ischemia or reperfusion injury) is important to avoid known contributors to secondary injury, such as hypotension.13,14 Accurate neuroprognostication is important to guide caregiver discussions and decision-making. Finally, given the elevated risk of neurodevelopmental impairment in cardiac arrest survivors, early referral for rehabilitation assessment and intervention is key.
A single Chain of Survival that supports the paradigm of early recognition through recovery after cardiac arrest has now been standardized across infants, children, and adults (outside of neonatal care; Figure 1).
Treatment of Respiratory Failure
Respiratory failure occurs when a patient’s breathing becomes inadequate and results in ineffective oxygenation and ventilation. This can occur due to disordered control of breathing, upper airway obstruction, lower airway obstruction, respiratory muscle failure, or parenchymal lung disease. Providing assisted ventilation when breathing is absent or inadequate, relieving FBAO, and administering naloxone in opioid overdose can be lifesaving.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Unless a cervical spine injury is suspected, use a head tilt–chin lift maneuver to open the airway in infants and children. |
| 1 | C-EO | 2. For infants and children with suspected cervical spinal injury, use a jaw thrust without head tilt to open the airway. |
| 1 | C-EO | 3. For infants and children with suspected cervical spinal injury, if the jaw thrust does not open the airway, use a head tilt–chin lift maneuver. |
Synopsis
Ensuring a patent airway is crucial for effective ventilation and oxygenation. Although there is no definitive evidence favoring a particular technique related to creating/maintaining patency of the airway, it is important for rescuers to understand the benefits and limitations of each method and to remain skilled in their application. Multiple approaches may be necessary to open an airway, and continuous monitoring of the pediatric patient is essential to confirm and maintain airway patency to ensure sufficient ventilation and oxygenation. Research comparing airway management strategies in pediatric cardiac arrest patients is lacking, with most available data derived from radiographic studies obtained during elective surgeries. As observed in the 2020 Guidelines, there continues to be a lack of pediatric-specific clinical studies. Figure 2 demonstrates the head tilt–chin lift maneuver to open the airway in infants.
Recommendation-Specific Supportive Text
- No data directly address the ideal method to achieve or maintain pediatric airway patency. One retrospective cohort study evaluated various head-tilt angles in neonates and young infants undergoing diagnostic magnetic resonance imaging and found that the highest proportion of patent airways was at a head-tilt angle of 144° to 150° based on a regression analysis.1
- While no pediatric studies evaluate jaw thrust versus head tilt to open the airway, jaw thrust is widely accepted as an effective way to open the airway, and this maneuver theoretically limits cervical motion compared with the chin lift.
- There are no pediatric studies evaluating the impact of a head tilt–chin lift maneuver to open the airway in a trauma patient with suspected cervical spine injury. However, if rescuers are unable to open the airway and deliver effective ventilations using a jaw thrust, given the importance of a patent airway, using a head tilt–chin lift maneuver is recommended.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For infants and children with a pulse but absent or inadequate respiratory effort, breaths should be provided. |
| 2a | C-EO | 2. For infants and children with a pulse but absent or inadequate respiratory effort, it is reasonable to give 1 breath every 2 to 3 s (20–30 breaths/min). |
Synopsis
The priority in managing a seriously ill or injured child who is not in cardiac arrest is evaluating the airway and breathing. If the child demonstrates signs of inadequate or absent breathing, breaths should be initiated to restore adequate oxygenation and ventilation. Because respiratory conditions are a major cause of cardiac arrest in infants and children, it is important to begin appropriate interventions to support ventilation and oxygenation quickly.
Recommendation-Specific Supportive Text
1. and 2. Pediatric-specific clinical studies evaluating the effect of different ventilation rates on outcomes in patients who have inadequate breathing with a pulse are lacking. In 2020, the guidelines were updated to reflect new data from a multicenter observational study that found that ventilation rates of at least 30/min in infants and at least 25/min in children greater than 1 year of age during CPR with an advanced airway in place were associated with improved return of spontaneous circulation (ROSC), survival to discharge, and survival with favorable neurological outcome. Excessive ventilation rates were associated with lower systolic blood pressures in older children, so care must be taken to avoid higher rates that could compromise hemodynamics.1 For the ease of training, the recommended respiratory rate for the pediatric patient with inadequate breathing and a pulse continues to be 1 breath every 2 to 3 seconds.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Infants or children with mild FBAO should be allowed to clear the airway by coughing while being observed for signs of severe FBAO. |
| 1 | C-LD | 2. For infants with severe FBAO, repeated cycles of 5 back blows alternating with 5 chest thrusts should be performed until the object is expelled or the infant becomes unresponsive. |
| 1 | C-LD | 3. For children with severe FBAO, repeated cycles of 5 back blows alternating with 5 abdominal thrusts should be performed until the object is expelled or the child becomes unresponsive. |
| 1 | C-LD | 4. If infants or children with severe FBAO become unresponsive, rescuers should start CPR, beginning with chest compressions (rescuers should not perform pulse check). |
| 1 | C-LD | 5. For infants or children with FBAO receiving CPR, rescuers should remove any visible foreign body when opening the airway to provide breaths. |
| 2b | C-LD | 6. Effectiveness and safety of suction-based airway clearance devices have not been established in infants and children. There is insufficient evidence to make a recommendation for infants and children. |
| 3: Harm | C-LD | 7. Blind finger sweeps should not be performed for infants and children with FBAO. |
Synopsis
Suffocation (eg, FBAO) is a leading cause of death in infants and children. Balloons, certain foods (eg, hot dogs, nuts, grapes), and small household objects are the most common causes of FBAO in children,1-3 while liquids are common among infants.4 It is important to differentiate between mild FBAO (the patient is coughing and making sounds) and severe FBAO (the patient cannot make sounds). Patients with mild FBAO can attempt to clear the obstruction by coughing, but intervention is required in severe obstruction. There are no high-quality data to support recommendations regarding FBAO in children. Figures 3 and 4 demonstrate chest thrusts and back blows in an infant with severe FBAO, and Figure 5 demonstrates abdominal thrusts in a child with severe FBAO. Figures 6 and 7 are the algorithms for infant and child FBAO.
Recommendation-Specific Supportive Text
1 and 3. Many FBAOs are relieved by allowing the patient to cough or, if severe, are treated by bystanders using chest thrusts or abdominal thrusts.4-8 A recent observational study of adult and pediatric FBAO suggests improved clearance of a foreign body with the use of back blows over abdominal thrusts8. To create consistency for instructional purposes, and in the absence of inferiority from pediatric data, management of severe FBAO in children now starts with a series of back blows instead of abdominal thrusts. Repeated cycles of 5 back blows followed by 5 abdominal thrusts are performed until the obstruction is cleared or the child becomes unresponsive.
2. Observational data primarily from case series support the use of back blows4,5,9-11 or chest thrusts7-9,12 for infants. Abdominal thrusts are not recommended for infants, given the potential to cause abdominal organ injury.13 The heel of hand technique for chest thrusts is now recommended for infants with severe FBAO, as current CPR literature suggests that it generates greater compression depth than the previously recommended 2-finger technique.14 While the heel of hand technique for chest thrusts resembles chest compressions that are used as part of CPR, there is no focus on the other components of high-quality CPR chest compressions (eg, rate, recoil) so the term chest compression is not used. If infants and children develop severe FBAO, emergency medical services should be promptly activated as they can rapidly deteriorate into cardiac arrest.
4. Once the infant or child is unconscious, observational data support immediate CPR with provision of chest compressions (do not perform a pulse check), followed by ventilations.8,12 Note that these compressions are delivered with attention to the provision of high-quality chest compressions as part of CPR.
5 and 7. Observational data suggest that the risk of blind finger sweeps outweighs any potential benefit in the management of FBAO.7,15-17
6. A solitary case series describes the use of portable non-powered suction devices for infants and children with severe FBAO.18 As the available evidence comes from voluntary reporting to an industry-sponsored registry, there is potential for significant confounding and bias, as well as incomplete case descriptions and patient outcomes reporting. The limited reported evidence also does not suggest superiority of these devices over standard techniques such as back blows or abdominal thrusts.
Sequence of Resuscitation
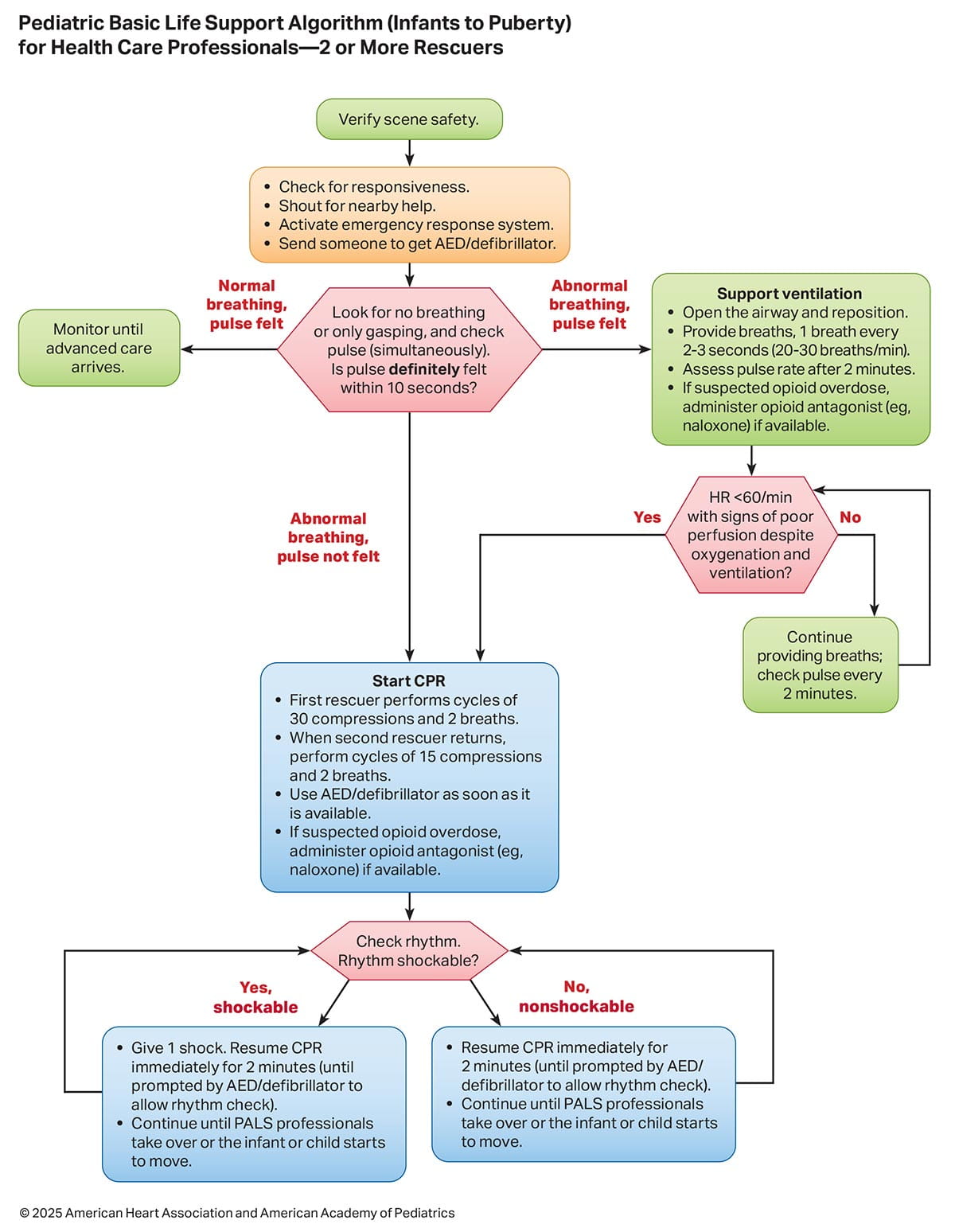
The sequence of resuscitation events is displayed in Figures 8, 9, and 10. Figure 8 is an infographic showing the critical actions for lay rescuers (ie, without medical knowledge or experience). Figures 9 and 10 are algorithms showing the critical sequences for those with a basic level of medical knowledge and experience. The use of opioid antagonists (eg, naloxone) during respiratory or cardiac arrest is incorporated into the BLS algorithms (Figures 9 and 10), with more detailed information provided in “Part 10: Adult and Pediatric Special Circumstances in Resuscitation.”12
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Lay rescuers should begin CPR immediately for any infant or child who is unresponsive, not breathing normally, and does not have signs of life; they should not check for a pulse. |
| 2a | C-LD | 2. In infants and children with no signs of life, it is reasonable for health care professionals to check for a pulse for up to 10 s and begin compressions unless a definite pulse is felt. |
| 2b | C-LD | 3. It may be reasonable to initiate CPR with compressions-airway-breathing over airway-breathing-compressions. |
Synopsis
Rapid recognition of cardiac arrest, immediate initiation of high-quality chest compressions, and delivery of effective ventilations are critical to improve outcomes from pediatric cardiac arrest. Lay rescuers should not delay starting CPR in a child with no “signs of life.” Palpation for the presence or absence of a pulse is not reliable as the sole determinant of cardiac arrest and the need for chest compressions. In infants and children, asphyxial cardiac arrest is more common than cardiac arrest from a primary cardiac event; therefore, effective ventilation is paramount during resuscitation of children. When CPR is initiated, the compressions-airway-breathing sequence mirrors the adult sequence to enhance educational simplicity in training.
Recommendation-Specific Supportive Text
- Lay rescuers are unable to reliably determine the presence or absence of a pulse and should not delay the initiation of CPR for any victim without signs of life.1-15
- No clinical trials have compared manual pulse checks with observations of “signs of life.” However, adult and pediatric studies have identified a high error rate, and harmful CPR pauses during manual pulse checks by health care professionals.16-19 In one study, health care professionals’ pulse palpation accuracy was 78%16 compared with lay rescuer pulse palpation accuracy of 47% at 5 seconds and 73% at 10 seconds.1
- One pediatric study demonstrated only a small delay (5.74 seconds) in commencement of breaths with compressions-airway-breathing compared with airway-breathing-compressions.20 Although the supporting evidence is minimal, continuing to recommend compressions-airway-breathing likely results in minimal delays in giving breaths and allows for a consistent approach to cardiac arrest treatment in adults and children.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Conventional CPR (chest compressions with breaths/ventilations) should be provided to infants and children in cardiac arrest. |
| 1 | B-NR | 2. For infants and children in cardiac arrest, interruptions in CPR should be minimized, and pauses in chest compressions should be less than10 s. |
| 1 | C-EO | 3. Rescuers should allow the chest to recoil completely after each compression. |
| 2a | B-NR | 4. If lay rescuers are unable or unwilling to deliver breaths to an infant or child in cardiac arrest, it is reasonable to provide compression-only CPR. |
| 2a | C-LD | 5. It is reasonable to use a chest compression rate of 100 to 120/min for infants and children. |
| 2a | C-LD | 6. For infants and children, it is reasonable for rescuers to provide chest compressions that depress the chest at least one-third the anterior-posterior diameter of the chest, which equates to approximately 1.5 in (4 cm) in infants and 2 in (5 cm) in children. |
| 2a | C-EO | 7. For health care professionals, it is reasonable to perform a rhythm check, lasting no more than 10 s, approximately every 2 min. |
| 2a | C-EO | 8. It is reasonable to provide ventilations with 100% oxygen during CPR. |
| 2a | C-EO | 9. When performing CPR without an advanced airway, it is reasonable for single rescuers to provide a compression-to-ventilation ratio of 30:2 and for 2 rescuers to provide a compression-to-ventilation ratio of 15:2. |
Synopsis
High-quality CPR generates blood flow to vital organs and increases the likelihood of ROSC. The 5 main components of high-quality CPR are (1) adequate chest compression depth, (2) optimal chest compression rate, (3) minimizing interruptions in CPR (ie, maximizing chest compression fraction or the proportion of time that chest compressions are provided for cardiac arrest), (4) allowing complete chest recoil between compressions, and (5) avoiding excessive ventilation. Compressions of inadequate depth and rate,1,2 incomplete chest recoil,3 and high ventilation rates4,5 are common during pediatric resuscitation.
Recommendation-Specific Supportive Text
- Despite increasing frequency of compression-only CPR in adults and children and mixed results when comparing conventional CPR (chest compressions and breaths) and compression-only CPR, large observational studies of children with OHCA show the best outcomes with conventional CPR.1-12 A small observational study of school-aged children found similar outcomes between conventional and compression-only CPR.12
- While 2 observational studies and a secondary analysis of a prospective multi-institutional randomized trial found that chest compression fraction (the percent of time during the CPR event where chest compressions were ongoing) of greater than 70% to 90% could be achieved in a majority of resuscitations, the association between a set chest compression fraction threshold to achieve best pediatric outcomes is unclear.13-15 There are inadequate data to recommend a goal chest compression fraction threshold for high-quality CPR in infants and children. However, two studies from a multinational, multi-institutional observational cohort registry showed that increased frequency and duration of pauses in CPR were significantly associated with lower probability of ROSC.16,17 Additionally, longer duration of pauses at the end of CPR prior to cannulation for extracorporeal life support were associated with poor neurologic outcomes and lower survival.17
- Allowing complete chest re-expansion improves the flow of blood returning to the heart, and therefore by increasing preload, increases blood flow to the body during CPR. There are no pediatric studies evaluating the effect of incomplete re-expansion during CPR, although leaning during pediatric CPR is common.18-20 In one observational study of invasively monitored and anesthetized children, leaning was associated with elevated cardiac filling pressures, leading to decreased coronary perfusion pressures during sinus rhythm.21
- Large observational studies of children with OHCA show that compression-only CPR is superior to no-bystander CPR.3-4,12,22
- A small observational study found that a compression rate of at least 100/min was associated with improved systolic and diastolic blood pressures during CPR for pediatric IHCA.23 One multicenter, observational study of pediatric IHCA demonstrated increased systolic blood pressures with chest compression rates between 100 and 120/min when compared with rates exceeding 120/min.24 Rates <100/min were associated with improved survival compared to rates of 100 to 120/min; however, the median rate in this slower category was approximately 95/min (ie, very close to 100/min).24
- Three anthropometric studies have shown that the pediatric chest can be compressed to one-third of the anteroposterior chest diameter without damaging intrathoracic organs.25-27 An observational study found an improvement in rates of ROSC and 24-hour survival, when at least 60% of 30-second epochs of CPR achieve an average chest compression depth >5 cm (about 2 inches) for pediatric IHCA in children.29
- Once an AED or monitor is available, a pause in chest compressions every 2 minutes allows for a brief rhythm check as well as allowing the change of personnel performing chest compressions to avoid fatigue.
- There are no human studies addressing the effect of varying inhaled oxygen concentrations during CPR on outcomes in infants and children. Given that the etiology of pediatric cardiac arrest is respiratory in nature, the use of 100% oxygen is physiologically appropriate.
- The optimum compression-to-ventilation ratio is uncertain. Large observational studies of children with OHCA demonstrated better outcomes with compression-ventilation CPR with ratios of either 15:2 or 30:2 compared with compression-only CPR.1,2
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For infants, rescuers should compress the sternum using either the 1-hand technique (heel of one hand on sternum) or the 2 thumb–encircling hands technique. If the rescuer cannot physically encircle the chest, it is recommended to compress the chest with the heel-of-1-hand technique. |
| 2b | C-LD | 2. For children, it may be reasonable to use either a 1- or 2-hand technique to perform chest compressions. |
Synopsis
In pediatric patients, due to the wide variation in the size of each patient, effective chest compression performance can be highly variable and depends on multiple factors, including the size and position of the rescuer as well as the hand position during CPR. Numerous studies have sought to determine optimal compression technique for each patient population.1-4 Prior recommendations were based solely on manikin studies for the optimal compression technique in the various pediatric age groups. However, new evidence from patient registries has led to new outcome-based recommendations.5 See Figure 11 for the 1-hand technique and Figure 12 for the 2 thumb–encircling hands technique for infants, and see Figure 13 for the 2-hand technique for children.
Recommendation-Specific Supportive Text
- In infants, systematic reviews and meta-analyses from simulation studies suggest that the 2 thumb–encircling hands technique is a superior technique when compared with 2-finger compressions, particularly for depth.6-10 In a multicenter prospective observational registry study, the 1-hand technique resulted in greater compression depth than the 2 thumb–encircling hands technique in infants with no difference in chest compression rate between hand positions.5 The 2-finger technique was utilized rarely in this study but, when used, no chest compression segments were compliant with AHA guidelines. An earlier single-center study did not show differences in chest compression depth between 1-hand and 2 thumb–encircling hands technique but noted 1-hand chest compressions were associated with an inappropriately fast rate.11
- In children 1 to 8 years of age, the 2-hand compression technique resulted in greater compression depth than the 1-hand technique.5 A secondary study from the same prospective observational registry showed that the 1-hand technique had better compliance with guideline chest compression rates than the 2-hand technique.5 There are no clinical data to determine if the 1-hand or 2-hand technique is associated with better outcomes for children receiving CPR. In manikin studies, the 2-hand technique has been associated with improved compression depth12 and compression force13 and less rescuer fatigue.14,15 More vertical compression angle (i.e., force applied closer to 90° perpendicular to the chest) to improve depth and recoil,16 with an "elbow-lock" technique while delivering chest compressions to maintain consistent vertical force and minimize fatigue,17 and the use of a stepstool during CPR5 have been studied as modifications to improve compression depth and reduce rescuer fatigue.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. It is reasonable to perform chest compressions on a firm surface in infants and children. |
| 2a | C-LD | 2. During IHCA, it is reasonable to use a backboard to improve chest compression depth in infants and children. |
Synopsis
Chest compression depth can be optimized by placing the child in a supine position on a hard, flat surface.1 It is challenging to perform adequate chest compressions on soft surfaces, such as a mattress, because both the chest and the surface itself can be compressed. In manikin studies, up to 57% of the compression force may be absorbed by the mattress leading to inadequate compression depth and increased rescuer fatigue.2–4 Backboard placement, use of the CPR mode on specially equipped hospital beds to return them to a flat position at a low height, and deflating air mattresses quickly or moving patients to the floor have been proposed to counter the impact of soft surfaces.5
Recommendation-Specific Supportive Text
- Chest compression depth is overall inadequate with few studies reaching the recommended targeted depth.6 Use of a firm surface in meta-analyses of manikin studies and a single pediatric case series show that compression depth is increased on a firm surface.7–9
- Meta-analysis of 7 studies4,10–15 showed a 2 mm (95% CI, 0.5–4 mm) increase in chest compression depth when CPR was performed on a manikin on a hospital mattress with a backboard in place.9
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In infants and children, CPR should be performed until the AED/manual defibrillator is ready to deliver a shock. |
| 1 | C-EO | 2. Prompt defibrillation with a single shock followed by immediate CPR, starting with compressions, is recommended for infants and children with VF/pVT. |
| 1 | C-EO | 3. Peri-shock pauses should be minimized during CPR in infants and children. |
Synopsis
Shockable rhythms are rare in infants and children. The risk of shockable rhythms, VF and pVT, steadily increases throughout childhood and adolescence, but remains less frequent than in adults. Shockable rhythms may be the initial rhythm of the cardiac arrest (primary VF/pVT) or may develop during the resuscitation (secondary VF/pVT). Defibrillation is the definitive treatment for VF/pVT. The shorter the duration of VF/pVT, the more likely that the shock will result in a perfusing rhythm. Minimizing frequency and duration of pauses in CPR for shock delivery improves chances of favorable short- and long-term outcomes.
Recommendation-Specific Supportive Text
- There are currently no pediatric data available regarding the optimal timing or duration of CPR prior to defibrillation. Adult studies show no benefit of a prolonged period of CPR before initial defibrillation.1-4
- Adult studies comparing a stacked shock (1-shock protocol versus a 3-shock protocol) for treatment of VF suggest a significant survival benefit with the single-shock protocol.5,6 The effectiveness of single compared to sequential (stacked) shocks has not been evaluated in infants and children with a shockable rhythm7, and therefore it is reasonable to give a single defibrillation dose of monophasic or biphasic energy followed by immediate resumption of chest compressions.6,8
- Prolonged pauses in chest compressions before and after shock delivery decrease blood flow and oxygen delivery to vital organs, such as the brain and heart, and are associated with decreased survival.9,10
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. When using an AED on infants and children less than 8 years of age, use of a pediatric attenuator is recommended. |
| 2a | C-LD | 2. For infants and children under the care of a trained health care professional, it is reasonable to use a manual defibrillator when a shockable rhythm is identified. |
| 2b | C-EO | 3. If neither a manual defibrillator nor an AED equipped with a pediatric attenuator is available, an AED without a pediatric attenuator may be considered for infants and children. |
Synopsis
Manual defibrillators and AEDs can both be used to treat VF/pVT in children. AEDs have high specificity in recognizing pediatric shockable rhythms.1-4 Manual defibrillators, when used by health care professionals, can be used to titrate energy dose to a patient's weight. Their use also minimizes pauses for rhythm analysis. Use of monophasic defibrillators has fallen out of favor because biphasic defibrillators require less energy with fewer side effects. Many AEDs are equipped to attenuate (reduce) the energy dose to make them more suitable for infants and children.
Recommendation-Specific Supportive Text
- Although there are no direct comparisons between pediatric attenuator and non-attenuator AED-delivered shocks, multiple studies document shock success with survival when a pediatric attenuator was used.1-13 A retrospective cohort study in children less than 18 years of age with OHCA showed that return of an organized rhythm was more likely when lower energy dose was utilized.14
- In adults, use of an AED in hospitals did not improve survival,15 and the peri-shock CPR pauses needed for rhythm analysis were prolonged.16 In children <18 years of age with IHCA, 1 well-designed retrospective multi-center cohort study, found that 30% of defibrillations were delivered for non-shockable rhythms with manual defibrillators,17 while previous studies suggest that AEDs misclassify pediatric shockable rhythms only 2% to 4% of the time. However, the adverse impact of shocking non-shockable rhythms in IHCA is unknown, and manual defibrillators allow for titration of energy dose to a patient’s weight while minimizing peri-shock CPR pauses compared to AED-associated CPR pauses.
- AEDs without pediatric attenuation deliver 120 J to 360 J, exceeding the recommended dose for children weighing <25 kg. However, there are reports of safe and effective AED use in infants and young children when the dose exceeds 2 to 4 J/kg.7-9,11,18,19 Because defibrillation is the only effective therapy for shockable rhythms, an AED without a dose attenuator may be lifesaving.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In infants and children, it is recommended to use the largest paddles or self-adhering pads that will fit on the chest while still maintaining good separation between the paddles or pads. |
| 2b | C-LD | 2. When using self-adhering pads in infants and children, either anteroposterior or anterolateral placement may be reasonable. |
| 2b | C-LD | 3. When delivering electric current for defibrillation in infants and children, paddles or self-adhering pads may be considered equally effective and efficient. |
Synopsis
Incorrect defibrillator paddle or pad placement can lead to ineffective delivery of electric current to the heart and adversely impact return of a perfusing rhythm. The size of the defibrillator paddles or self-adhering pads depends on the age of the patient. Paddles or self-adhering pads of the largest size that can still be separated by at least 1 to 2 cm allow for appropriate current flow. When using paddles, an electrical conducting interface is needed to reduce skin and thoracic impedance. Figures 14 and 15 demonstrate placement of the pads in the anteroposterior position, and Figure 16 demonstrates placement of the pads in the anterolateral (AL) position).
Recommendation-Specific Supportive Text
- Minimizing transthoracic impedance is important to optimize delivery of electric current. Studies comparing shocks delivered to children with pediatric to adult paddles showed that transthoracic impedance was significantly higher with pediatric paddles due to smaller size.1-3
- One pediatric study demonstrated no difference in rates of ROSC with anteroposterior compared to anterolateral pad placement.4 Among adults, a prospective cohort study showed statistically higher adjusted odds of ROSC at any time with anteroposterior pad placement while another study showed no significant difference.5,6
- One simulation study demonstrated that the predominant factor to prompt defibrillation was rescuer familiarity with the defibrillator and the paddles/pads connections. There was no significant difference in median time to shock with use of paddles compared with self-adhering pads.7

Figure 15. Placement of pediatric pads in the alternative anteroposterior position.
One pad is placed on the left side of the chest, between the left side of the child’s breastbone and left nipple (anterior) and the other pad is placed on the left side of the child’s back, next to the spine (posterior).

Figure 16. Placement of pediatric pads in the anterolateral position.
The right anterior pad is placed vertically on the right chest, with the top of the pad just below the clavicle. The left lateral pad is placed horizontally on the left side of the chest, centered at the mid-axillary line, with the top of the pad on the lower ribcage in line with the sternal border (inferior portion of the xyphoid process). Do not place the pad on breast tissue or the abdomen.
Critical Knowledge Gaps and Ongoing Research
During the literature review process, we identified several critical knowledge gaps related to pediatric basic and advanced life support. These topics are either current areas of ongoing research or lack significant pediatric evidence to support evidence-based recommendations. In addition, we identified topics for which systematic or scoping reviews are in process by the ILCOR BLS or Pediatric Life Support Task Forces and elected not to make premature recommendations until these reviews are available.
As is so often the case in pediatric medicine, many recommendations are extrapolated from adult data. This is particularly true for the BLS components of pediatric resuscitation. The causes of pediatric cardiac arrest are very different from cardiac arrest in adults, and pediatric studies are critically needed. Furthermore, infants, children, and adolescents are distinct patient populations. Dedicated pediatric resuscitation research is a priority given the more than 20,000 infants, children, and adolescents who suffer cardiac arrest in the United States each year.
Critical knowledge gaps are summarized in Table 2.
Table 2. Critical Knowledge Gaps Due to Insufficient Pediatric Data
| What frequency should the rhythm be checked during CPR? |
| What is the optimal ventilation rate during CPR in patients with or without an advanced airway? Is it age dependent? |
| What is the optimal chest compression rate during CPR? Is it age dependent? |
| What is the optimal CCF for optimal outcomes in pediatrics? |
| What is the optimal position for performing chest compressions (eg, on a stepstool, kneeling on the bed, standing)? |
| What is the effect of leaning on the chest and providing incomplete re-expansion of the chest in between chest compressions? |
| What is the ideal concentration of oxygen to deliver when performing CPR? |
| What is the ideal compression-to-ventilation ratio? |
| What is the optimal timing and dosing of defibrillation for VF/pVT? |
| What clinical tools can be used to help in the decision to terminate pediatric IHCA and OHCA resuscitation? |
| What is the optimal timing or duration of CPR prior to defibrillation? |
| What duration of pause in chest compressions is “prolonged” pause? Or, what is the duration of time that is considered a minimal pause in chest compressions? |
| What is the effectiveness of single shock compared to sequential (stacked) shocks in pediatrics? |
| What is the adverse impact of shocking non-shockable rhythms with a manual defibrillator in IHCA? |
| Is the time to shock with self-adhesive pads significantly different than with paddles? |
| Is there an optimal pad position for defibrillation in pediatrics? If so, is it AP or AL? |
| What is the ideal threshold of CCF associated with improved patient outcomes in pediatric CPR? |
| What is the most effective intervention for FBAO in pediatric patients, chest compressions versus abdominal thrusts? |
| What is the safety and efficacy of commercial devices for FBAO? |
| What is the role of mechanical chest compression devices in pediatric patients? |
| What is the role of point-of-care ultrasound (vascular or cardiac) during rhythm and pulse analysis? |
| What is the best method to verify effectiveness of breaths/ventilations during rescue breathing and CPR? |
AL indicates anterolateral; AP, anteroposterior; CCF, chest compression fraction; CPR, cardiopulmonary resuscitation; FBAO, foreign body airway obstruction; IHCA, in-hospital cardiac arrest; OHCA, out-of-hospital cardiac arrest; pVT, pulseless ventricular tachycardia; and VF, ventricular fibrillation.
The American Heart Association requests that this document be cited as follows: Joyner BL, Dewan M, Bavare A, de Caen A, DiMaria K, Donofrio-Odmann J, Fosse G, Haskell S, Mahgoub M, Meckler G, Requist J, Schexnayder SM, Olech Smith M, Werho D, Raymond TT. Part 6: pediatric basic life support: 2025 American Heart Association and American Academy of Pediatrics Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2025;152(suppl2):S424–S447. doi: 10.1161/CIR.0000000000001370
This article has been copublished in Pediatrics.
- Benny L. Joyner, Jr., MD, MPH, Co-Chair
- Maya Dewan, MD, MPH
- Aarti Bavare, MD, MPH
- Allan de Caen, MD
- Kimberly DiMaria, DNP, CPNP-AC
- Joelle Donofrio-Odmann, DO
- Gwen Fosse, MSA, BSN, RN
- Sarah Haskell, DO
- Melissa Mahgoub, PhD
- Garth Meckler, MD, MSHS
- Jennifer Requist, BS, RRT-NPS
- Stephen M. Schexnayder, MD
- Michelle Olech Smith, DNP, MS, RN
- David Werho, MD
- Tia T. Raymond, MD, MBA, Co-Chair
The writing group would like to thank the Emergency Cardiovascular Care Pediatric Emphasis Group members for their contributions: Janice A. Tijssen, MD; Jonathan P. Duff, MD, MEd; Ryan W. Morgan, MD, MTR; Brian Jackson, MD; Candace N. Mannarino, MD, MS; Georg M. Schmölzer, MD, PhD; Javier J. Lasa, MD; Jennifer Hayes, RN, MSN; Jessica Fowler, MD, MPH; Katherine Remick, MD; Marc Auerbach, MD, MSc; Paul Mullan, MD; Todd Chang, MD; Tehnaz Boyle, MD, PhD; Vishal Kapadia, MD, MSCS; Farhan Bhanji, MD, MSc.
Open table in a new window.
Open table in a new window.
 Login
Login