Part 10: Adult and Pediatric Special Circumstances of Resuscitation
Abstract
In these guidelines, the American Heart Association provides updated guidance for resuscitation of adults and children in cardiac arrest or with a life-threatening condition due to special circumstances, including anaphylaxis, asthma, cardiac arrest in the cardiac intervention suite, cardiac arrest following cardiac surgery, drowning, electrocution, gas embolism, high consequence respiratory pathogens, hyperkalemia, hyperthermia, hypothermia, left ventricular assist device failure, pregnancy, pulmonary embolism, and poisoning due to benzodiazepines, β-blockers, calcium channel blockers, cocaine, cyanide, digoxin and related cardiac glycosides, local anesthetic systemic toxicity, methemoglobinemia, opioids, organophosphates and carbamates, sodium channel blockers, sympathomimetics, and volatile hydrocarbons. Recommendations are also provided for alternatives to cardiopulmonary resuscitation and the use of extracorporeal membrane oxygenation for poisoned patients. Adults and children with these conditions require modification of basic life support and advanced life support. These guidelines are based on systematic evidence reviews and provide separate graded recommendations for adults and children.
Top 10 Take-Home Messages
- Anaphylaxis: Isotonic intravenous (IV) fluids may be used for fluid resuscitation in cardiac arrest from anaphylaxis, whereas standard anaphylaxis dose of epinephrine administered via autoinjector or intramuscularly may not offer benefit. Glucagon may be reasonable to administer in cases refractory to standard treatment when β-blocker exposure is suspected. Extracorporeal membrane oxygenation (ECMO) is reasonable in refractory cases.
- Cardiac interventional laboratory: Some adults in cardiac arrest in the cardiac interventional laboratory may require specialized interventions, including performing a corrective procedure to treat the etiology of the arrest, mechanical cardiopulmonary resuscitation (CPR), extracorporeal life support (ECLS), or intracoronary epinephrine.
- ECLS: While ECLS is not available in every setting, adults and children in cardiac arrest or a peri-arrest state with a potentially reversible etiology may be supported with ECLS devices, such as venoarterial ECMO (VA-ECMO), in disease processes such as anaphylaxis, asthma, cardiac surgery, cardiac interventional laboratory, hypothermia, and pulmonary embolism (PE) and in poisonings like β-blockers, calcium channel blockers (CCBs), cocaine, local anesthetics, sodium channel blockers, and sympathomimetics.
- High-consequence respiratory pathogen: Chest compressions, bag-mask ventilation, defibrillation, suctioning, and endotracheal intubation should be considered aerosol-generating procedures, which pose a risk of infection to resuscitation team members. However, a real-world study found that rates of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) transmission to resuscitation team members using personal protective equipment (PPE) were low.
- Hyperkalemia: Clinical evidence supporting IV calcium or IV sodium bicarbonate administration is limited in humans and uncertain to improve survival or favorable neurological outcomes. The utility of other therapies intended to lower potassium concentrations in the setting of cardiac arrest is unclear when weighing their possible benefits against the risk of harm if well-established interventions, such as CPR, are interrupted.
- Hyperthermia: Adults and children with life-threatening hyperthermia from environmental causes, cocaine poisoning, or sympathomimetic poisoning should be rapidly cooled, ideally at a rate of at least 0.15 °C/min (0.27 °F/min). This is best achieved with immersion in ice water.
- Hypothermia: Adults and children with life-threatening environmental hypothermia may survive with good neurological outcomes even after prolonged cardiac arrest. Patients should be rewarmed concurrently with resuscitation efforts. ECLS can be used where available.
- Left ventricular assist device (LVAD): The absence of a palpable pulse can make confirming cardiac arrest in adults and children with an LVAD difficult, thus perfusion is assessed using skin color, skin temperature, capillary refill, mean arterial pressure, and partial pressure of end-tidal carbon dioxide. Treatment includes prioritization of CPR while simultaneously assessing and attempting to restart LVAD function if a second rescuer is available.
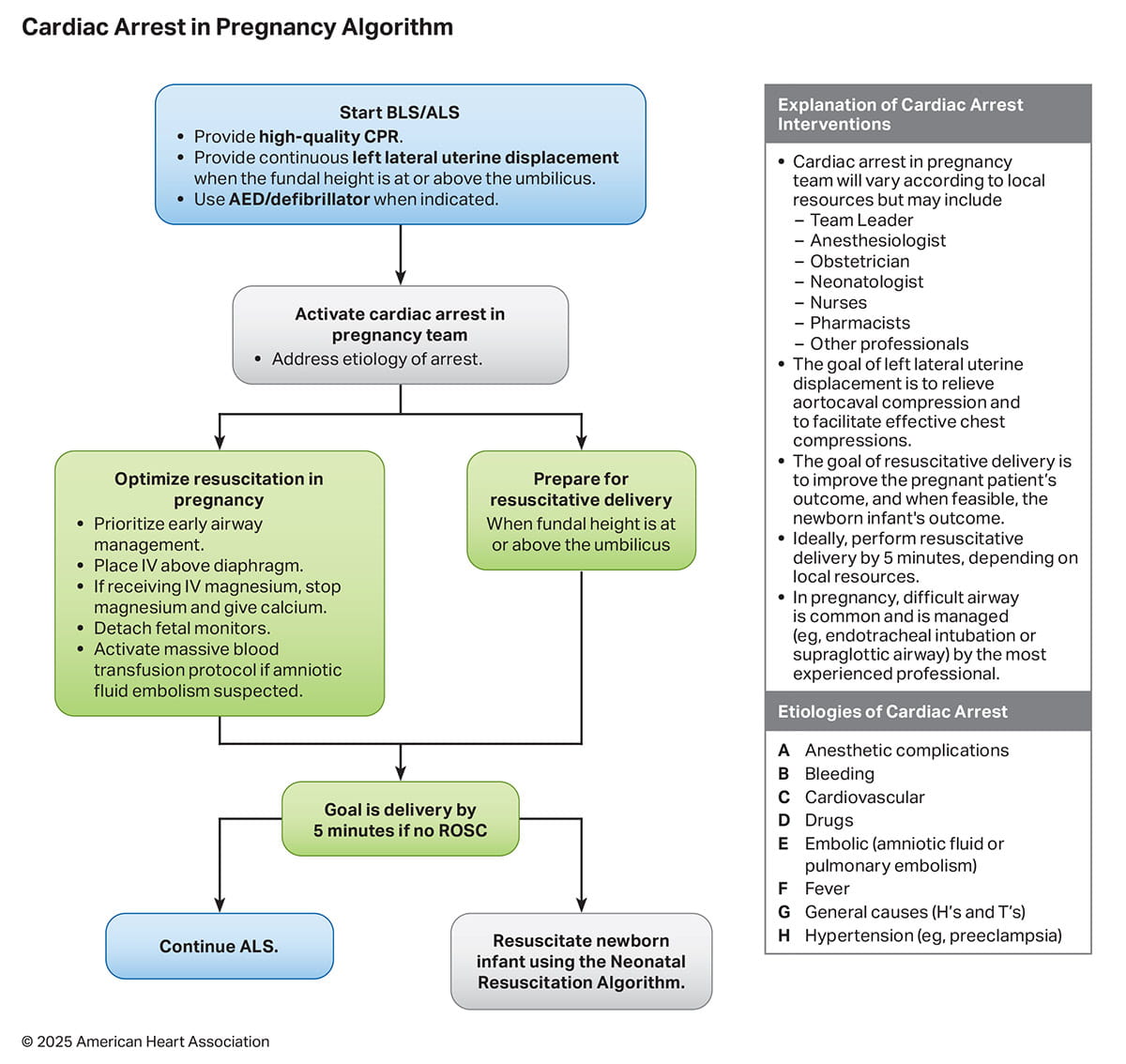
- Pregnancy: Management of cardiac arrest during pregnancy is a complex clinical scenario that requires resuscitation strategies that accommodate for the physiological changes of pregnancy. Resuscitation priorities include early airway management and left lateral uterine displacement.
- Opioids: An opioid antagonist (eg, naloxone) should be given to people with respiratory arrest from suspected opioid overdose. Trained rescuers, lay rescuers, and members of the general public can all administer naloxone. During resuscitation of cardiac arrest due to presumed opioid overdose, an opioid antagonist may be reasonable to administer if high-quality CPR is not interrupted.
Preamble
The chain of survival is a series of steps in the process of cardiac arrest management that begins with prevention and concludes with survivorship. Throughout, coordinated efforts are required of laypeople and health care professionals in a variety of disciplines across the health care system. These guidelines contain recommendations for basic life support (BLS) and advanced life support (ALS) for adults and children in special circumstances resulting in cardiac arrest and are based on the best available resuscitation science. For these recommendations, children are defined as those without signs of puberty in the BLS setting and those who are less than 18 years of age in the ALS setting. Depending on the clinical data immediately available, there are numerous special circumstances in which additional interventions or modifications to BLS and ALS in adults and children may be required, spanning each link in the chain of survival. Management of patients in cardiac arrest from these special circumstances often differs from standard resuscitation. For example, patients may develop hypotension from β-adrenergic receptor antagonist (eg, β-blocker) or calcium channel antagonist (eg, CCB) poisoning that does not respond to atropine, standard vasopressors, or cardiac pacing but is amenable to targeted therapies, such as high-dose insulin. In addition, specific recommendations about the training of resuscitation professionals are provided in “Part 12: Resuscitation Education Science” and recommendations about systems of care are provided in “Part 4: Systems of Care.”
Scope of the Guidelines
These guidelines are designed primarily for North American health care professionals treating adults and children in cardiac arrest or a life-threatening state from a special circumstance requiring modification of BLS and ALS. Life-threatening conditions include a range of medical emergencies that pose an immediate risk to survival (eg, respiratory arrest, refractory hypotension, critical metabolic acidosis), while a cardiac arrest refers to the cessation of cardiac output and, with that, cessation of oxygen delivery throughout the body. Depending on the data available, recommendations are made for the management of patients in cardiac arrest or for patients with life-threatening conditions inclusive of cardiac arrest. Additionally, for certain topics, the relevant literature regarding the management of cardiac arrest in these settings may be limited to patients in a life-threatening condition. The same therapies are recommended for life-threatening conditions and cardiac arrest unless otherwise stated.
These guidelines contain recommendations for BLS and ALS for both adults and children. Unless otherwise specified, the interventions recommended here are intended for use in addition to standard BLS, pediatric BLS, ALS, and pediatric ALS (PALS) resuscitation. Although many of these treatments are impractical outside of the hospital setting, several can be initiated by emergency medical services and some (eg, giving breaths to drowning victims) may be relevant to lay rescuers. These guidelines are intended to be used in conjunction with topic-specific references and advice from local and regional experts.
The special circumstances guidelines have historically been developed from predominantly adult data. As is common in pediatric medicine, these recommendations were often applied to the resuscitation of children in similar contexts. For the 2025 recommendations, the literature searches performed included all age groups, and for which pediatric data are available, recommendations were made in line with those data. For those where there are sparse to no pediatric data, the recommendations will be absent or extrapolated from adult data.
Throughout these guidelines, recommendations may include therapies including procedures that all centers are not sufficiently resourced to offer, such as ECLS, hyperbaric oxygen therapy, and mechanical thrombectomy. These therapies may, however, be available for select centers and in those settings should be implemented or considered depending on the strength of the evidence and the clinical context. It is important for health care professionals to follow their institutional guidelines as well as the available literature.
Organization of the Writing Committee
The writing group included a diverse group of experts with backgrounds in emergency medicine, pediatric emergency medicine, adult and pediatric critical care, anesthesiology, adult and pediatric cardiology, electrophysiology, obstetrics and gynecology, maternal fetal medicine, hyperbaric medicine, medical toxicology, pharmacy, trauma care, education, and research. Group members were appointed by the American Heart Association (AHA) Emergency Cardiovascular Care Science Subcommittee. Each recommendation was developed and formally approved by the writing group and subsequently reviewed and approved by the AHA Emergency Cardiovascular Care Science Subcommittee.
The AHA has rigorous conflict-of-interest policies and procedures to minimize the risk of bias or improper influence during the development of guidelines. Before appointment, writing group members disclosed all relevant commercial relationships and other potential (including intellectual) conflicts. These procedures are described more fully in Part 2: Evidence Evaluation and Guidelines Development in the 2025 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Writing Group Disclosures at the end of this document lists the writing group members’ relevant relationships with industry (Appendix 1(link opens in new window)).
Methodology and Evidence Review
The writing group members first created and approved a list of special circumstances topics, drawing on the scope of prior guidelines and new topics that have gained prominence since the 2020 publication. A population, intervention, comparison, outcome, study design, and time frame (PICOST) question was created for each topic. Guided by the writing group chairs and with assistance from a professional medical librarian as needed, the writing group performed a structured evidence evaluation for each topic, which was internally peer reviewed. These searches were executed in Medline and the Excerpta Medica Database (Embase) using the Ovid search interface, and the Cochrane Central Register of Controlled Trials. International Liaison Committee on Resuscitation (ILCOR) evidence reviews published since 2020 were reviewed, and the dates of updated searches were harmonized with these reviews to avoid search overlap. Final complete searches were executed in July 2024 with single database searches in November through December 2024. Structured searches were supplemented by bibliography review and ad hoc searches when needed. Search results were imported into Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). At least 2 writing group members performed dual screening of the titles and abstracts of all articles identified from each search and identified articles for full-text review. Screening conflicts were resolved between the 2 writing group members and writing group leadership before full-text review. Two writing group members reviewed the full text of all selected articles and applied the information contained to develop treatment recommendations appropriate for each clinical question. Each draft recommendation was created by a group of 2 writing group members and then reviewed and refined by all writing group members during regular virtual meetings and 2 in-person meetings. Completed draft recommendations were reviewed by organizational leaders in the AHA with recommendations incorporated as draft revisions. Final draft recommendations were then externally peer reviewed. A more comprehensive description of these methods is provided in “Part 2: Evidence Evaluation and Guidelines Development.”
Class of Recommendation and Level of Evidence
Each recommendation was assigned a Class of Recommendation (COR) based on the strength and consistency of the evidence, alternative treatment options, and impact on patients and society (Table 1). Recommendation wording flows in a structured manner based on the COR determination. The Level of Evidence (LOE) is based on the quality, quantity, relevance, and consistency of the available evidence. For each recommendation, the writing group discussed and approved specific recommendation wording and the COR and LOE assignments. In determining the COR, the writing group considered the LOE and other factors, including systems issues, economic factors, and ethical factors, such as equity, acceptability, feasibility, and risk of harm. These evidence review methods, including specific criteria used to determine COR and LOE, are described more fully in “Part 2: Evidence Evaluation and Guidelines Development” of the 2025 Guidelines.
Despite improvements in the design and funding support for emergency care research, the overall quality of the evidence for special circumstances science is very low. Only 2 of the 235 recommendations in these guidelines are supported by Level A evidence (high-quality evidence from more than 1 randomized controlled trial [RCT] or 1 or more RCTs corroborated by high-quality registry studies). Nine recommendations are supported by Level B randomized evidence (moderate evidence from 1 or more RCTs) and 32 by Level B nonrandomized evidence. The majority of recommendations are based on Level C evidence, including those based on limited data (67 recommendations) and expert opinion (125 recommendations). Accordingly, the strength of recommendations is weaker than optimal; 66 Class 1 (strong) recommendations, 84 Class 2a (moderate) recommendations, and 58 Class 2b (weak) recommendations are included in these guidelines. In addition, 21 recommendations are designated Class 3: No Benefit and 6 recommendations are Class 3: Harm. Clinical trials, thoughtfully designed intervention studies with real-world applicability, and well-controlled observational studies in special circumstances science are needed.
Open table in a new window.
Guideline Structure
These guidelines are organized into modular knowledge chunks grouped into discrete modules of information on specific topics or management issues. Each modular knowledge chunk includes a table of recommendations that uses standard AHA nomenclature of COR and LOE. A brief introduction is provided for topics with multiple sets of recommendations, and a synopsis may also be written to put the recommendations into context with important background information and overarching management or treatment concepts. Recommendation-specific supportive text clarifies the rationale and key study data supporting the recommendations. When appropriate, figures, flow diagrams, and additional tables are included.
Document Review and Approval
All guidelines were reviewed and approved for publication by the AHA Emergency Cardiovascular Care Science Advisory Committee, the AHA Scientific Advisory and Coordinating Committee, and the AHA Executive Committee. These guidelines were also submitted for blinded peer review to subject matter experts nominated by the AHA. Before appointment, all peer reviewers were required to disclose relationships with industry and any other conflicts of interest, and all disclosures were reviewed by AHA journal staff. Comprehensive disclosure information for peer reviewers is listed in the Reviewer Disclosure Table (Appendix 2(link opens in new window)).
These recommendations supersede the last full set of AHA special circumstances guidelines, published in 2020, and recommendations from the focused updates on toxicology and drowning.1-3
To maintain consistency across the different chapters of the guidelines, the LOE for some recommendations that were previously assigned LOE B-NR (nonrandomized) or C-LD (limited data) based on uncontrolled observational studies, case reports, and nonhuman studies were modified to C-EO (expert opinion). Supporting data are provided in the recommendation-specific supportive text.
Overview
Survival and recovery from cardiac arrest depend on a complex system working together to achieve the best outcomes. The main focus of treatment of adults and children in cardiac arrest includes rapid recognition, prompt provision of CPR, defibrillation of malignant shockable rhythms, adequate ventilation, post–return of spontaneous circulation (ROSC) supportive care, and treatment of underlying causes. This approach recognizes that cardiac arrest is due to either primary cardiac causes, such as myocardial infarction and electric disturbances, or without a primary cardiac origin, such as respiratory failure or toxic ingestion. In such cases, treatment for reversible underlying causes is important for the rescuer to consider. For any cardiac arrest, rescuers are instructed to call for help, perform CPR with breaths, and apply an automated external defibrillator (AED) to directly treat ventricular fibrillation (VF) or ventricular tachycardia (VT), if present. Although the majority of resuscitation success is achieved by the provision of high-quality CPR and defibrillation, other specific treatments for likely underlying causes may be helpful in some cases.
These guidelines, focusing on the special circumstances as etiologies of cardiac arrest, provide and evaluate specific treatment options meant to be administered in addition to, and alongside, traditional resuscitation care. Unless otherwise specified, all patients should receive standard airway management, support of breathing, and treatment of hypotension, arrhythmias, and cardiac arrest consistent with local protocols and the resources available at the site of treatment.
In addition to defibrillation, several alternative and pseudoelectrical therapies have been explored as possible treatment options during cardiac arrest. Precordial thump is a single, sharp, high-velocity impact (or “punch”) to the middle of the sternum delivered by the ulnar aspect of a tightly clenched fist. A precordial thump can generate an electric impulse, theoretically interrupting a life-threatening tachyarrhythmia or “kick-starting” the heart in asystole.1 However, if a precordial thump is administered during the electrically vulnerable portion of an organized rhythm (T wave), like an unsynchronized shock or commotio cordis, there is a risk of deterioration of cardiac rhythm. Percussion (fist) pacing is the delivery of serial, rhythmic, relatively low-velocity impacts to the sternum by the ulnar aspect of a closed fist. This is administered to stimulate an electric impulse sufficient to cause myocardial depolarization and electrically pace the heart in cases of asystole or a life-threatening bradyarrhythmia.1 Cough CPR involves a deep breath followed immediately by a cough, repeated every few seconds, to provide transient hemodynamic support at the onset of a hemodynamically significant arrhythmia in a patient who is both conscious and cooperative. Interposed abdominal compression CPR is a 3-rescuer technique that involves conventional chest compressions combined with alternating abdominal compressions. The dedicated rescuer who provides manual abdominal compressions will compress the abdomen midway between the xiphoid and the umbilicus during the relaxation phase of chest compression. Alternative techniques for performing interposed abdominal compression involve use of compression devices. One such device consists of pressure application handles, a compression plate, and a display panel showing compression force, and a second device uses a seesaw-like function, causing abdominal decompression during sternal compression and vice versa.2,3 These devices are not currently available in North America and are therefore outside the scope of these recommendations.
Recommendations for precordial thump, precordial pacing, and interposed abdominal compression in adults and children inform the management of adults and children in cardiac arrest. Recommendations for cough CPR inform the management of adults and children who have life-threatening arrhythmias but are conscious and able to follow commands.
| COR | LOE | Recommendations |
|---|---|---|
| 3: No Benefit | B-NR | 1. A precordial thump should not be performed on adults in cardiac arrest. |
| 3: No Benefit | C-EO | 2. A precordial thump should not be performed on children in cardiac arrest. |
Recommendation-Specific Supportive Text
- The use of a precordial thump during routine cardiac arrest care in the out-of-hospital setting did not improve survival to hospital discharge compared with standard CPR in adults in 2 nonrandomized studies.4,5 Defibrillation, as a first-line intervention, is significantly more likely to result in ROSC.4 In adults, precordial thump resulted in immediate ROSC in some cases of in-hospital cardiac arrest (IHCA) and out-of-hospital cardiac arrest (OHCA) but also led to rhythm degeneration, such as from VT to VF or asystole.4,6-9 The majority of reports of successful use of precordial thump are for ventricular arrhythmias in adults with IHCA with success more likely in cases with VT than VF.7,9-15 There are also cases of a precordial thump successfully causing ROSC in adults with asystole.5,6 There are reports of successful use of a precordial thump for restoration of cardiac output in adult cases of induced ventricular tachyarrhythmias in the inpatient cardiology setting, but the majority of cases failed to respond.16-19 Across studies, there is a lack of standardization in the technique of precordial thump, the number of times it was used, pharmacological therapy administered before or after its delivery, and—in some cases—its timing related to the onset of the arrhythmia.
- There are no studies on the use of precordial thump during cardiac arrest in children. Extrapolating from data for adults, it can be stated that precordial thump does not improve outcomes compared with standard ALS in OHCA, and there are no comparative studies for IHCA. There are case reports of serious complications due to the use of precordial thump in children.4,5,20
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-EO | 1. Percussion (fist) pacing may be considered in adults as a temporizing measure in the witnessed, monitored, in-hospital (eg, cardiac intervention laboratory or intensive care unit [ICU]), onset of asystole or a nonperfusing bradyarrhythmia before loss of consciousness and if performed without delaying definitive therapy. |
| 3: No Benefit | C-EO | 2. Percussion pacing should not be performed routinely in adults in cardiac arrest. |
| 3: No Benefit | C-EO | 3. Percussion pacing should not be performed in children in cardiac arrest. |
Recommendation-Specific Supportive Text
- Limited evidence from adult descriptive studies demonstrates effective use of percussion pacing during witnessed inpatient asystolic or life-threatening bradycardic events with favorable outcomes of survival to hospital discharge, ROSC, or restoration of cardiac output.21-25
- No controlled or comparative studies show that the use of percussion pacing improves outcomes compared with standard therapy in adults. It would not be appropriate to prioritize percussion pacing over other measures with proven efficacy.25
- No controlled or comparative studies show that the use of percussion pacing improves outcomes compared with standard therapy in children. Two pediatric cases show successful generation of a QRS complex and pulse with percussion pacing.25,26 It would not be appropriate to prioritize percussion pacing over other measures with proven efficacy.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. Cough CPR may be reasonable as a temporizing measure in the witnessed, monitored, in-hospital (eg, cardiac intervention laboratory or ICU), onset of a hemodynamically significant tachyarrhythmia or bradyarrhythmia before loss of consciousness and if performed without delaying definitive therapy in adults. |
| 3: No Benefit | C-EO | 2. Cough CPR should not be performed routinely by adults with life-threatening arrhythmias. |
| 3: No Benefit | C-EO | 3. Cough CPR should not be performed by children with life-threatening arrhythmias. |
Recommendation-Specific Supportive Text
- Some evidence suggests that cough CPR increases aortic and left ventricular pressures.27,28 Observational studies describe the use of cough CPR in conscious adults at the witnessed onset of several arrhythmias (VT, VF, high-degree atrioventricular [AV] blocks, severe bradycardia, and asystole) with successful termination and prevention of loss of consciousness.6,27-29 In 1 study of 115 adults with Morgagni-Adams-Stokes syndrome and frequent fainting episodes during arrhythmias, patients were trained in cough CPR. On follow-up, 66 patients applied cough CPR in a total of 365 events, 80% of which were reported as effective, but these occurred in an unmonitored setting so the cardiac rhythm at the time of symptoms is unknown.28 Importantly, while cough CPR may be reasonable in specific circumstances, it may divert time, effort, and attention from clinicians performing high-quality CPR or patients seeking medical attention.
- No controlled or comparative studies show that use of cough CPR improves outcomes compared with standard therapy in adult patients. The risks are (1) that it delays effective treatment (early call for help, early CPR and defibrillation if the patient loses consciousness and stops breathing normally) and (2) that members of the public who confuse cardiac arrest with heart attack delay seeking help when suffering chest pain or other symptoms indicating a possible ischemic cardiac event. An untrained adult is unlikely to be able to reliably identify a cardiac arrest rhythm in time to initiate coughing to maintain a cardiac output. It would not be appropriate to prioritize cough CPR over other measures with proven efficacy.
- There are no studies of cough CPR in children. There is risk of delaying effective treatment, and younger children are unlikely to understand or correctly perform the technique. It would not be appropriate to prioritize cough CPR over other measures with proven efficacy.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | B-R | 1. The effectiveness of interposed abdominal compression CPR is uncertain in adults. |
| 2b | C-EO | 2. The effectiveness of interposed abdominal compression CPR is uncertain in children. |
Recommendation-Specific Supportive Text
- Two early, relatively small, unblinded adult RCTs of interposed abdominal compression CPR compared with standard CPR for IHCA done at a single institution during different time periods showed improved ROSC, short-term survival, and survival to hospital discharge.30,31 Two recent, relatively small, unblinded adult RCTs of interposed abdominal compression CPR in IHCA showed no difference in ROSC.32,33 Two RCTs of adult OHCA did not show any increase in ROSC.34,35 More evaluation is needed to define the optimal clinical scenario for this technique.
- There are no studies on the use of interposed abdominal compression CPR in children. More evaluation is needed to define the optimal clinical scenario for this technique.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO | 1. It is reasonable to fluid resuscitate adults and children in cardiac arrest from anaphylaxis with isotonic IV fluids. |
| 2a | C-EO | 2. It is reasonable to use extracorporeal CPR (ECPR) for adults and children in cardiac arrest from anaphylaxis refractory to pharmacological interventions. |
| 2b | C-EO | 3. It may be reasonable to administer glucagon to adults and children who are in cardiac arrest from anaphylaxis with suspected or confirmed concurrent β-blocker use and who are refractory to standard ALS or PALS medications. |
| 2b | C-EO | 4. For adults and children in cardiac arrest from rocuronium-induced anaphylaxis, the effectiveness of sugammadex is uncertain. |
| 2b | C-EO | 5. For adults and children in cardiac arrest from anaphylaxis, the effectiveness of standard anaphylaxis intramuscular (IM) epinephrine dose is uncertain. |
Synopsis
Anaphylaxis represents a severe multisystem immune modulated reaction to a substance that can occur across all age groups. Common triggers include insects, foods, and medications, such as anesthetics, antibiotics, blood products, and chemotherapeutics. Perioperative anaphylaxis can be challenging to diagnose and has resulted in delayed treatment.1 Anaphylaxis after neuromuscular blockade resulting in cardiac arrest has been associated with high mortality (34.7%) even when ALS guidelines are followed.2 Acute coronary syndrome resulting from a rare allergic reaction, also known as Kounis syndrome, with patient-specific complexities is beyond the scope of this review.3
Immune activation can manifest systemically, most commonly affecting the skin, gastrointestinal, pulmonary, and cardiovascular systems. The rapidity of onset and progression to airway occlusion with potential cardiovascular collapse make timely diagnosis and treatment essential for positive outcomes.
Options for treatment of anaphylaxis in the peri-arrest setting include epinephrine (IM or IV), antihistamines, bronchodilators, corticosteroids, glucagon, and discontinuation or removal of the trigger.
Our review did not identify any interventional studies for cardiac arrest from anaphylaxis. All reviewed studies were observational studies of anaphylaxis, which included subgroups with cardiac arrest. These recommendations inform the management of adults and children in cardiac arrest from anaphylaxis.
Recommendation-Specific Supportive Text
- Intravascular volume depletion secondary to fluid extravasation is a reversible cause of cardiac arrest. The use of isotonic fluid resuscitation for cardiac arrest from anaphylaxis is supported only by case studies. In 1 adult case report, 73% of administered fluid had been reported to extravasate to the extravascular space via third spacing and increased vascular permeability during anaphylaxis.4 In a non–cardiac arrest clinical trial of adult patients with anaphylaxis, patients had more rapid improvement in symptoms with IV fluids.5 There is no data specific to children.
- Evidence for ECPR in the setting of anaphylaxis is limited to case reports. In 1 case series of 6 patients (5 adults and 1 adolescent), only 1 of the 4 adults in cardiac arrest received ECPR.6 The patient survived and had a favorable neurological outcome. In a case series of 6 adults with cardiac arrest from anaphylaxis following succinylcholine exposure, only 1 received ECPR but died after 12 days.7
- Indirect evidence from anaphylaxis-induced hypotension in adult case reports supports the administration of glucagon (1–5 mg IV in adults, 20–30 mcg/kg IV push up to 1 mg in children) in the treatment of refractory anaphylaxis in patients with concurrent β-blocker use or suspected exposure. 8-10 Administration should not preclude standard ALS or PALS protocols. There is no data specific to children.
- In an adult observational study of severe anaphylaxis after exposure to neuromuscular blocking agents, a history of cardiovascular disease other than hypertension was identified as a risk factor for death from cardiac arrest.2 The survival from cardiac arrest in this study was 156/199 (78%). Case reports exist suggesting sugammadex as a potential treatment for anaphylaxis associated with rocuronium. However, a case series in adults did not show improvement in outcomes of anaphylaxis without cardiac arrest.11 A second study in adults found decreased vasopressor requirements in 4 of 7 patients who received sugammadex.1 There are no data specific to children
- Extrapolation of findings from studies in adults and children indicates that the bioavailability and time to peak blood concentration for equal-dose (eg, mg/kg) IM epinephrine is inferior to IV and intraosseous (IO) routes of administration. When comparing pharmacokinetics across different studies, anaphylaxis dose IM epinephrine had lower and delayed peak epinephrine concentrations as compared to IV epinephrine in cardiac arrest.12-16 There is limited high-quality data directly comparing IV or IO epinephrine with IM epinephrine, including no data for cardiac arrest secondary to anaphylaxis. In a before-and-after implementation study of an early, first-dose IM epinephrine (5 mg) for 1405 adults with OHCA compared with usual care, IM epinephrine was associated with improved survival to hospital admission (adjusted odds ratio [aOR], 1.37; 95% CI, 1.06–1.77), hospital survival (aOR, 1.73; 95% CI, 1.10–2.71), and favorable neurologic status at hospital discharge (aOR, 1.72; 95% CI, 1.07–2.76).17 Notably, the dose of epinephrine administered by the IM route was much higher than standard ALS or PALS IV or IO dosing and standard dosing for anaphylaxis management.18 In cardiac arrest from anaphylaxis, standard anaphylaxis IM epinephrine dose may be insufficient.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In adults and children with life-threatening asthma, sudden elevation in peak inspiratory pressures or difficulty ventilating should prompt evaluation for tension pneumothorax. |
| 2a | C-LD | 2. It may be reasonable to use ECLS for adults and children with life-threatening asthma refractory to standard therapies. |
| 2a | C-LD | 3. A ventilation strategy of low respiratory rate and tidal volume is reasonable in adults with life-threatening asthma. |
| 2a | C-EO | 4. A ventilation strategy of low respiratory rate and tidal volume is reasonable in children with life-threatening asthma. |
| 2b | C-LD | 5. Treatment with volatile anesthetics for adults and children with life-threatening asthma refractory to standard therapies may be considered. |
| 2b | C-EO | 6. Active exhalation maneuvers in adults and children with life-threatening asthma refractory to standard therapies may be considered. |
Synopsis
Asthma can lead to cardiac arrest primarily from increased lower airway obstruction leading to hypoxemia, hypercarbia, respiratory acidosis, and increased intrathoracic pressure leading to decreased cardiac output.1 Care of the patient in the setting of an acute asthma exacerbation includes bronchodilators, steroids, and noninvasive ventilation and in cardiac arrest standard resuscitation.2 There are insufficient data to comment on the use of magnesium or ketamine in patients with life-threatening asthma.
These recommendations inform the management of adults and children with life-threatening asthma exacerbation, including cardiac arrest.
Recommendation-Specific Supportive Text
1. Tension pneumothorax is a rare life-threatening complication of asthma and a potentially reversible cause of cardiac arrest. Although tension pneumothorax usually occurs in mechanically ventilated patients, cases are also reported in spontaneously breathing adults and children.3-5 High peak airway pressures resulting from positive-pressure ventilation can lead to pneumothorax. While difficulty ventilating an adult or child with asthma in extremis is more likely due to hyperinflation and high intrathoracic pressure, evaluation for tension pneumothorax remains important.3-5
2. Multiple observational studies in adults and children have demonstrated the use of ECLS in the context of respiratory failure from asthma refractory to standard care.6-13 Survival rates in these studies are 83.5% to 100%. Most patients in these studies were supported with venovenous ECMO; however, patients with hemodynamic instability in addition to persistent hypoxia, hypercarbia, or barotrauma may require VA-ECMO support.
3. and 4. Acute respiratory failure can precipitate cardiac arrest in adults and children with asthma, which is characterized by severe obstruction leading to air trapping. Because of the limitation in exhalation air flow, delivery of large tidal volumes at a higher respiratory rate can lead to progressive worsening of air trapping and a decrease in effective ventilation. An approach described in adult asthma patients using lower tidal volumes, lower respiratory rate, and increased expiratory time may minimize the risk of intrinsic positive end-expiratory pressure and barotrauma.14,15 This approach may be extrapolated to children with life-threatening asthma.
5. Several observational studies in adults and children have demonstrated the use of volatile anesthetics in the context of respiratory failure from asthma refractory to bronchodilators, steroids, intubation, mechanical ventilation, sedation, and neuromuscular blockade.7,10,16-20 Survival ranged from 87% to 100% in these studies. Two of these studies compared ECLS versus volatile anesthetics.7,10 These studies did not show a significant difference in mortality but had mixed results in other clinical endpoints, such as length of stay, complication rate, and occurrence of patient procedures.
6. Adults and children with severe asthma have limited ability to exhale and have a phenomenon known as breath stacking. This, in turn, can lead to an increase in intrathoracic pressure, decrease in venous return, decrease in blood pressure, decrease in coronary perfusion pressure, and, ultimately, cardiac arrest. Adults and children manifest with difficulty ventilating, high airway pressure during ventilation, or a sudden decrease in blood pressure. Brief disconnection from the ventilator or a pause in bag-mask ventilation and compression of the thorax to aid exhalation may relieve hyperinflation. This practice is informed by several case series with mixed conclusions. In 3 case series, adults and children received active exhalation maneuvers via external compression to relieve air trapping with improvement in clinical status.21-23 However, 1 case series of 4 adults showed no improvement with external compression during exhalation.24
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. It is reasonable to use mechanical CPR devices to provide chest compressions to adults in cardiac arrest in the cardiac intervention laboratory. |
| 2a | C-LD | 2. It is reasonable to use ECLS in adults in cardiac arrest in the cardiac intervention laboratory when initial therapy is failing. |
| 2b | C-LD | 3. It may be reasonable to use intracoronary epinephrine for adults in cardiac arrest in the cardiac intervention laboratory during percutaneous coronary intervention (PCI). |
Synopsis
Diagnostic and therapeutic cardiovascular procedures—such as coronary angiography, PCI, transcatheter valvular intervention, and catheter ablation—are performed in the cardiac intervention laboratory. Potential procedural risks include coronary or myocardial injury associated with coronary ischemia, pericardial effusion or cardiac tamponade, severe valvular dysfunction, or malignant arrhythmia, all of which can lead to cardiac arrest. Severe cardiovascular comorbidities are common in these patients, increasing the risk of cardiovascular complications.
Cardiac arrest during PCI has better survival than in other settings, including in-hospital units, likely because of vigilant monitoring, immediate defibrillator availability, established intravascular access, and rapid intervention to treat the cause of cardiac arrest.1-10
Many patients with cardiac arrest in the cardiac intervention laboratory will respond to standard advanced cardiovascular life support (ACLS). In some cases, high-quality CPR may interfere with corrective interventions (eg, pericardiocentesis) or vice versa.
These recommendations inform the management of adults in cardiac arrest and do not pertain to adults who are in cardiogenic shock without cardiac arrest or those who are in cardiac arrest prior to arrival in the cardiac interventional laboratory.
These recommendations are specific to adults because studies specific to children were not included in the literature review.
Recommendation-Specific Supportive Text
- Observational studies in adults have demonstrated the feasibility of using mechanical CPR devices for administering chest compressions to adults in cardiac arrest in the cardiac intervention laboratory.11-17 This allows performance of potentially lifesaving interventions, such as PCI or placement of mechanical circulatory support, while avoiding gaps in the performance of CPR. Most studies utilized a mechanical piston device, and 1 used a load-distributing band.11,13-17 Two studies on mechanical CPR in adults in cardiac arrest that specifically occurred in the cardiac intervention laboratory showed 25% survival with good neurological outcome, and 1 showed improved outcomes compared with manual CPR.16,17 In another comparative study including adults in cardiac arrest prior to arrival in the cardiac intervention laboratory, patients receiving mechanical chest compressions were more likely to achieve ROSC than those receiving manual chest compressions.15 However, both comparative studies were nonrandomized, had small sample sizes, and compared against a historical cohort, which coincided with major changes in resuscitation guidelines.15,16
- There are no comparative studies on the use of ECLS versus standard therapy for adults in cardiac arrest in the cardiac intervention laboratory. The recommendation for ECLS is informed by prospective observational studies.18-24 However, many studies include adults receiving ECLS for cardiac arrest that occurred prior to the cardiac intervention laboratory or ECLS use for cardiogenic shock without cardiac arrest. The Extracorporeal Life Support Organization (ELSO) Registry found survival to hospital discharge was 39% in patients receiving ECLS for cardiac arrest in the cardiac intervention laboratory, with a lower risk-adjusted odds of mortality in this population than in those with cardiac arrest receiving ECLS in an ICU or in a hospital bed.21 Notably, some patients in this study received mechanical circulatory support prior to cardiac arrest, which may confound outcome data. The largest study of adults who specifically had initial cardiac arrest in the cardiac intervention laboratory showed a 30-day survival of 44%.23 There are reports of successful use of standby ECMO in adults undergoing high-risk PCIs and transcatheter aortic valve replacements in the cardiac intervention laboratory.25,26 Standby ECMO (placement of small-bore vascular sheaths and preprimed ECMO circuits with appropriately sized cannulas ready prior to cannulation) was associated with lower low-flow time, lower rate of acute kidney injury, and improved 30-day survival compared with extemporaneous ECMO (no cannulation or preparation of an ECMO circuit prior to cardiac arrest) in high-risk PCI.25
- Two prospective cohort studies compared epinephrine (1 mg every 3–5 minutes) via intracoronary versus IV (peripheral or central) administration in 320 adults who developed cardiac arrest in the cardiac intervention laboratory.27,28 Both showed improved outcomes, such as ROSC and survival with favorable neurological outcome, with intracoronary epinephrine. However, both studies were limited by potential confounding because the route of epinephrine administration was at the physician’s discretion and due to some inherent differences between groups.
Cardiac arrest after cardiac surgery occurs in patients relatively infrequently, although these patients typically have better outcomes than those with other etiologies of cardiac arrest in different settings.1 Common causes of cardiac arrest in this setting include VF (typically secondary to ischemia or pacemaker malfunction), cardiac tamponade, and severe hypovolemia from bleeding. Unique to this setting is the highly monitored nature of the postoperative ICU as well as the potential for cardiac trauma with chest compressions given the recent surgical suture lines. This population also often has the potential for temporary pacing through epicardial wires attached at the time of the operation as well as the relative ease of performing emergent resternotomy and internal cardiac massage, which provides improved output compared with external chest compressions.2 The 2017 Society of Thoracic Surgeons (STS) consensus statement on this topic, including recommendations for children, minimally invasive heart surgeries, LVADs, transplant patients, and sternotomy and nonsternotomy operations, in addition to evidence review from the writing group informs these guidelines. Further, these societal guidelines are focused on the initial hospitalization from the operation itself, particularly during the ICU course.1 Refer to other relevant sections of the 2025 AHA guidelines for recommendations related to cardiac arrest during or immediately after cardiac catheterization (refer to Cardiac Intervention Laboratory) or cardiac arrest in patients with LVADs who are remote from the immediate postoperative period (refer to Left Ventricular Assist Devices).
These recommendations inform the management of adults and children in cardiac arrest after cardiac surgery.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For adults in cardiac arrest after cardiac surgery, external chest compressions should be performed if emergency resternotomy is not immediately available. |
| 1 | C-EO | 2. For adults in VF cardiac arrest after cardiac surgery, if a trained professional witnesses the cardiac arrest, immediate 3-stacked defibrillation should be performed, and CPR should be initiated if defibrillation is not successful within 1 minute. |
| 1 | C-EO | 3. For adults in asystole or a bradycardic arrest after cardiac surgery, if a trained professional witnesses the arrest and temporary pacing wires are already in place, immediate pacing is recommended and CPR should be initiated within 1 minute if pacing is unsuccessful. |
| 2a | B-NR | 4. For adults in cardiac arrest early after cardiac surgery, it is reasonable to perform rapid resternotomy in an appropriately staffed and equipped ICU. |
| 2a | B-NR | 5. Internal cardiac massage can be useful in adults after cardiac surgery if cardiac arrest develops when the chest or abdomen is open or in the early postoperative period after cardiothoracic surgery when the chest can be reopened rapidly. |
| 2a | C-EO | 6. For adults in cardiac arrest after cardiac surgery who are refractory to standard resuscitation, ECLS can be effective. |
Recommendation-Specific Supportive Text
- Chest compressions following cardiac surgery have the potential for cardiac trauma as demonstrated in adult case reports as well as cardiac trauma in others who have not had cardiac surgery.3,4 However, adult observational studies have not reported such trauma.5,6 External chest compressions remain the primary means of providing perfusion while the chest is closed. In these cases, the benefit of perfusion using external chest compressions outweighs the risk of potential cardiac trauma.
- VF is a common presenting rhythm in cases of cardiac arrest after cardiac surgery often secondary to ischemia or pacemaker malfunction. Immediate defibrillation presents distinct advantages in these adult patients, whereas the potential morbidity associated with external chest compressions or resternotomy may impact recovery.7 Limited adult data are available from defibrillator threshold testing with backup transthoracic defibrillation using variable waveforms and energy doses.8-10 First-shock success over 90% was observed in most of these studies, though pooled results from 15 studies found a defibrillation success rate of 78% for the first shock, 35% for the second, and 14% for the third.11 The STS Task Force on Resuscitation After Cardiac Surgery and the European Association for Cardio-thoracic Surgery recommend 3-stacked defibrillations within 1 minute, before initiation of CPR or resternotomy.1,7 This departure from standard ACLS is likely warranted in the post–cardiac surgery setting because of the highly monitored setting and unique risks of compressions and resternotomy.
- Immediate pacing of adults in asystole or a bradycardic arrest after cardiac surgery is supported by surgical societies.1,7 Available hemodynamic monitoring modalities in conjunction with manual pulse detection provide an opportunity to confirm myocardial capture and adequate cardiac function. When pacing attempts are not successful within 1 minute, standard ACLS including CPR is indicated.
- No RCTs of resternotomy have been published. Improved outcomes have been observed with rapid resternotomy protocols when performed by experienced health care professionals in an appropriately equipped ICU.5,6,12-18 Other observational studies show no benefit of resternotomy compared with standard ACLS therapy.19-23 The STS recommends that resternotomy be a standard part of the resuscitation protocols for at least 10 days after surgery.1 Resternotomy performed outside of the ICU results in poor outcomes.16,21
- A systematic review of external cardiac compressions compared with internal cardiac massage that included animal studies as well as 9 human studies consistently demonstrated improved cardiac index and coronary perfusion pressure with internal massage.1,2,12,21,24-29 The authors of the systematic review concluded that prompt conversion from external chest compressions to internal cardiac massage improves opportunities for ROSC.2 The STS expert consensus statement and the European Resuscitation Council (ERC) guidelines recommend converting to internal cardiac massage unless brief resuscitation with defibrillation, pacing, or medication is successful.2,7
- Many case series in adults, collectively inclusive of over 4000 patients, have reported rescue with ECLS (eg, ECMO, ventricular assist device, cardiopulmonary bypass [CPB]) in the setting of refractory cardiac arrest or persistent cardiogenic shock after cardiac surgery with a survival range of 36% to 78%.30-34 Cannulation for cardiogenic shock and cardiac arrest post cardiotomy has been demonstrated to be feasible, although with a low survival rate (11%) in adults.35
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For children in cardiac arrest after cardiac surgery, external chest compressions should be performed if emergency resternotomy is not immediately available. |
| 2a | C-LD | 2. For children in cardiac arrest early after cardiac surgery, it is reasonable to perform resternotomy early in an appropriately staffed and equipped ICU. |
| 2a | C-LD | 3. For children in cardiac arrest after cardiac surgery who are refractory to standard resuscitation procedures, ECLS can be effective. |
| 2a | C-EO | 4. Internal cardiac massage can be useful in children after cardiac surgery if cardiac arrest develops when the chest or abdomen is open or in the early postoperative period after cardiothoracic surgery when the chest can be reopened rapidly. |
Recommendation-Specific Supportive Text
- Chest compressions following cardiac surgery have the potential for cardiac trauma as demonstrated in adult case reports as well as cardiac trauma in others who have not had cardiac surgery.3,4,36 However, adult observational studies have not reported such trauma and external chest compressions remain the primary means of providing perfusion while the chest is closed.5,6 No evidence was identified specific to the pediatric population. While there is no specific pediatric data, extrapolating from adult literature to children in cardiac arrest following cardiac surgery, the benefit of perfusion using external chest compressions outweighs the risk of potential cardiac trauma.
- No RCTs of resternotomy have been published. Outcomes for resternotomy in the pediatric population are extrapolated from mixed positive and neutral studies in adults with rapid resternotomy protocols in an appropriately equipped ICU.5,6,12-20,22,23,37 One observational study including adults and children showed proportionally greater survival in children with resternotomy and open chest cardiac compression.21 The STS recommends that resternotomy be a standard part of the resuscitation protocols for at least 10 days after surgery.1 Resternotomy performed outside of the ICU results in poor outcomes.16,21
- One study compared children receiving external chest compressions and those receiving ECPR for cardiac arrest post–cardiac surgery.38 Those who received ECPR had an odds ratio for survival of 2.2. Additionally, many case series in children have reported rescue with ECLS (eg, ECMO, ventricular assist device, CPB) in the setting of refractory cardiac arrest or persistent cardiogenic shock after cardiac surgery with a survival range of 36% to 78%.38-46
- A systematic review of external cardiac compressions compared with internal cardiac massage that included animal studies as well as 9 human studies consistently demonstrated improved cardiac index and coronary perfusion pressure with internal massage.1,2,12,21,25,27,28 One of the 9 human studies included 34 children in the analysis.21 The authors of the systematic review concluded that prompt conversion from external chest compressions to internal cardiac massage improves opportunities for ROSC.2 The STS expert consensus statement and the ERC guidelines recommend converting to internal cardiac massage unless brief resuscitation with defibrillation, pacing, or medication is successful.2
Drowning is the third leading cause of death from unintentional injury worldwide, accounting for 7% of all injury-related deaths.1 Physical trauma, such as head injuries, spinal injuries, or fractures from accidents (eg, diving and boating accidents), can impair a person’s ability to remain conscious, swim, or stay afloat, increasing the risk of drowning. Drowning generally progresses from initial respiratory arrest due to submersion-related hypoxia to cardiac arrest; thus, it can be challenging to distinguish respiratory arrest from cardiac arrest because pulses are difficult to accurately palpate within the recommended 10-second window. Therefore, resuscitation from cardiac arrest attributable to this specific circumstance must focus on restoring breathing as much as it does circulation.2
These guidelines are designed for health care professionals, trained rescuers, and untrained lay rescuers resuscitating adults and children who have drowned. We have defined trained rescuer as an individual with appropriate training to perform the task discussed in a given recommendation. This is independent of the individual’s occupation or ethical duty to respond. Lifeguards, swimming instructors, emergency medical technicians, paramedics, police, firefighters, other volunteers, and off-duty health care professionals, if appropriately trained for the task mentioned in the recommendation, would be considered trained rescuers and would be expected to respond in the recommended manner.3 The Drowning Chain of Survival (Figure 1) highlights a series of interventions that reduce drowning-associated mortality when put into action by trained rescuers or untrained lay rescuers.2,4
We acknowledge the contributions of the authors of “2024 American Heart Association and American Academy of Pediatrics Focused Update on Special Circumstances: Resuscitation Following Drowning: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.”2,3
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. The effectiveness of artifact-filtering algorithms for analysis of electrocardiogram rhythms during chest compressions for adults in cardiac arrest has not been established. |
Synopsis
Whenever an unresponsive person is found in the water, the rescuer is confronted with a difficult choice of whether to initiate breaths immediately or once the drowned person has been removed from the water. The drowning process involves initial hypoxia that may progress to respiratory arrest followed by cardiac arrest if not promptly addressed.5-12
This recommendation informs the management of adults and children who are unresponsive after drowning and not necessarily confirmed in cardiac arrest.
Recommendation-Specific Supportive Text
- One retrospective observational study of in-water resuscitation of nonbreathing drowned adults and children rescued by lifeguards found higher odds of survival to hospital discharge and hospital survival with favorable neurological outcome compared with similar individuals who did not receive in-water resuscitation.12 Rescuers must consider personal safety, availability of equipment, and distance to shore when determining the appropriateness of performing in-water breaths. When respiratory arrest is addressed by breaths that interrupt the drowning process, the death rate is lower (44%) than in those cases that progress to cardiac arrest and receive CPR (93%).6,7,9-12
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Trained rescuers should provide supplemental oxygen if available to adults and children with cardiac arrest following drowning. |
Synopsis
Hypoxia is the major sequela of drowning and when severe and prolonged can lead to cardiac arrest. Even with effective CPR, cardiac output, cerebral oxygenation, and blood flow range from 12% to 42% of prearrest values.1-3 Additionally, there is decreased diffusion capacity of the lungs from aspiration. A full discussion of the pathophysiology of drowning is beyond the scope of this guideline.4 Adult BLS and pediatric BLS guidelines support the use of maximal available oxygen concentration during CPR (refer to “Part 6: Pediatric BLS” guidelines and “Part 7: Adult BLS” guidelines). Oxygen administration has regulatory and legal restrictions in several countries and requires the provision, use, and maintenance of equipment as well as an understanding of the mechanisms and risks of oxygen administration and storage. Use of oxygen may be limited in low- and middle-income countries. These recommendations inform the management of adults and children in cardiac arrest following drowning.
Recommendation-Specific Supportive Text
- No study directly addresses the use, timing, or concentration of oxygen delivery to drowned adults and children. The use of supplementary oxygen during and after CPR is accepted practice for trained rescuers providing resuscitation from drowning due to the hypoxic nature of the arrest.5,6 Hypoxemia is associated with decreased survival to hospital admission, and rapid reversal of hypoxemia through prompt bystander CPR is associated with improved survival to hospital admission and neurologically favorable survival from drowning.7-15 We do not make a recommendation about the use of pulse oximetry monitoring during drowning because of uncertainty regarding the effectiveness or the accuracy of pulse oximetry in the setting of CPR.16-19
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO | 1. It is reasonable for trained rescuers to provide breaths or ventilations by the first means available (eg, mouth-to-mouth, pocket mask, or bag-mask ventilation) for adults and children in cardiac or respiratory arrest following drowning to avoid any delay in ventilation. |
| 2a | C-EO | 2. It is reasonable to provide ventilations using equipment (bag-mask device or advanced airways) for adults in cardiac arrest following drowning. |
| 2a | C-EO | 3. It is reasonable to provide ventilations using equipment (bag-mask device or supraglottic airway) for children in cardiac arrest following drowning. |
| 2a | C-EO | 4. It is reasonable to provide rescuers with a competency-based training program with regular retraining and maintenance of equipment. |
Synopsis
Adequate ventilation is a priority during resuscitation in cardiac arrest following drowning.1,2 This can be achieved with the use of mouth-to-mouth breathing, pocket masks, or other equipment (bag-mask device, supraglottic airway, or endotracheal intubation) according to the rescuer’s training and availability of ventilatory equipment. In 30:2 CPR following OHCA, lung inflation with bag-mask ventilation is infrequent but is associated with improved ROSC, survival to discharge, and survival with favorable neurological outcomes.3 In OHCA in general, current guidelines support the use of bag-mask ventilation or an advanced airway (supraglottic airway or endotracheal tube), depending on the situation and the skill set of the rescuer (refer to “Part 8: PALS” and “Part 9: ALS” in these guidelines).3 Three RCTs comparing different airway strategies in adult OHCA yielded similar results when the rescuers were adept at advanced airway techniques (eg, endotracheal intubation, supraglottic airway) but inferior outcomes when they were not.4-6 These studies highlight the critical importance of effective ventilation by ensuring secondary measures, such as monitoring chest rise and fall.
These recommendations inform the management of adults and children who are either in respiratory arrest without cardiac arrest or who are in cardiac arrest following drowning.
Recommendation-Specific Supportive Text
- Providing breaths by the first means available is associated with improved survival to hospital discharge or 30 days for adults and children in cardiac arrest following drowning.7-10 No human drowning studies have directly compared the different methods of delivering breaths.1,2 Manikin studies enrolling lifeguards showed that mouth-to-mouth ventilation resulted in fewer chest compression interruptions and more effective ventilation and tidal volume delivery than the use of a pocket mask, bag-mask device, or face shield.11,12
- and 3. Providing ventilations with equipment has not been compared with no equipment during resuscitation following drowning.1,2 In 2 large observational studies of adults with cardiac arrest from drowning, there was no difference in survival with good neurological outcome between adults who had endotracheal intubation and those with supraglottic airway or bag-mask ventilation.13,14 In children with cardiac arrest from drowning, endotracheal intubation was associated with worse neurologic outcomes.15,16
- CPR education research demonstrates that providing effective breaths is a difficult skill to master. Training is most effective when it incorporates a hands-on aspect and is repeated to build retention.17-19
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For adults and children in cardiac arrest following drowning and after removal from the water, CPR with breaths and chest compressions should be provided. |
| 2a | B-NR | 2. For adults in cardiac arrest following drowning, if the rescuer is unwilling, untrained, or unable to provide breaths, it is reasonable to provide chest compressions only, until help arrives. |
| 2b | C-EO | 3. For children in cardiac arrest following drowning, if the rescuer is unwilling, untrained, or unable to provide breaths, it may be reasonable to provide chest compressions only, until help arrives. |
| 2b | C-EO | 4. For adults and children in cardiac arrest following drowning, it may be reasonable for trained rescuers to initiate CPR with breaths first followed by chest compressions. |
Synopsis
While these recommendations adhere to standard BLS principles, adults and children removed from the water without normal breathing or consciousness have important physiologic considerations that may modify standard algorithms. Cardiac arrest following drowning is most often due to a hypoxic mechanism, whereas sudden cardiac arrest, particularly in adults, is more likely to occur with fully oxygenated blood. An emphasis on breaths is highlighted in these recommendations.
These recommendations inform the management of adults and children in cardiac arrest following drowning.
Recommendation-Specific Supportive Text
- Multiple large observational studies of adults and children in cardiac arrest following drowning show improved outcomes when CPR included breaths.1-4
- and 3. One study including primarily elderly patients in hot tubs found no difference in outcomes between conventional (eg, CPR with breaths) and compression-only CPR. However, the demographics of the individuals who drowned in this study differed from those in most other drowning studies.5,6 Most cardiac arrests from drowning are hypoxic in nature, and breaths are important. However, the etiology of the arrest may be unknown and some individuals in cardiac arrest from drowning will have a primary cardiac etiology where compression-only CPR is reasonable. No pediatric data on compression-only CPR following drowning were identified, thus, the pediatric recommendation is extrapolated from the adult data.
- There is no direct evidence evaluating the sequence of resuscitation in cardiac arrest following drowning in adults or children (eg, airway-breathing-circulation versus circulation-airway-breathing). One small observational study found improved survival and neurological outcome among nonbreathing adults and children who may have had cardiac arrest following drowning and received immediate in-water breaths compared with delayed on-land resuscitation.7 A manikin study that was not specific to drowning found that the time to completion of first CPR cycle (30 compressions and 2 breaths) was 15 seconds shorter in a compression-first strategy compared with an airway and breathing–first strategy.8 Initiating CPR with airway, breathing, and chest compressions by trained rescuers may be appropriate as long as the initiation of breaths does not delay compressions (eg, waiting for ventilation equipment).
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For adults and children in cardiac arrest following drowning, CPR with breaths should be started before AED or defibrillator application. |
| 2a | B-NR | 2. AED use is reasonable for adults and children in cardiac arrest following drowning. |
Synopsis
Cardiac arrest following drowning is most commonly from a hypoxic drowning process; however, it less commonly may be from a primary cardiac event.1 Initial shockable rhythms constitute a minority (2%–12%) of cardiac arrests following drowning but are associated with higher odds of survival.2-8 Shockable rhythms following drowning may be less common because of longer durations of submersion or longer response times. The low incidence of shockable rhythm supports the emphasis placed on high-quality CPR with breaths elsewhere in this document.2,4,6 Individuals may also present with shockable cardiac arrest due to nondrowning causes in an aquatic setting. AEDs are feasible and safe to use in cardiac arrest following drowning.9,10 A trend toward more frequent application of AEDs in cardiac arrest following drowning has been demonstrated.11
These recommendations inform the management of adults and children in cardiac arrest following drowning.
Recommendation-Specific Supportive Text
- Observational studies in adults and children of OHCA, and specifically cardiac arrest following drowning, show improved survival to hospital discharge or 30 days when CPR including breaths were initiated promptly.8,12-14 AED application may present delays to CPR. In a study of 919 adult and children with cardiac arrest from drowning, where only 7.4% had an initial shockable rhythm, an AED was applied before emergency medical services arrival in 32.4% and was associated with a decreased likelihood of favorable neurological outcome in the adjusted analyses (aOR, 0.42; 95% CI, 0.23–0.77; P less than 0.005), which may have been due to a delay in the initiation of high-quality CPR with breaths and compressions.8
- It is difficult to quantify the benefit of AED use in cardiac arrest following drowning given that shockable rhythms occur less commonly in the drowning process. When shockable rhythms are present, AED application is practical and a shockable rhythm confers a survival benefit in studies of adults and children with cardiac arrest after drowning.4,6,8-11,15
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO | 1. Implementation of public access defibrillation programs is reasonable in areas where there is a high risk of cardiac arrest, including aquatic environments. |
Synopsis
Cardiac arrest can occur in high-use public areas, such as airports, parks, beaches, pools, and other areas with high population density, with frequent utilization, where other forms of exercise are performed, or with long distances or response time to the nearest AED. Early defibrillation is associated with increased odds of survival; therefore, ensuring public access to defibrillators is important. Public access defibrillation (PAD) programs have been associated with improved outcomes for OHCA.1,2 Although initial shockable rhythms from drowning are uncommon (occurring in 2%–12% of cases of cardiac arrest following drowning), for those who do have an initial shockable rhythm, early defibrillation can be lifesaving.3-6 PAD programs have been demonstrated to be cost-effective.7 Targeted AED placement compared with nationwide deployment has been demonstrated to have incremental cost-effectiveness; however, cost-effectiveness data for AED placement in aquatic environments are lacking. This recommendation applies only to the programmatic placement of AEDs in aquatic environments (eg, through public policies), not to their specific use in resuscitation following drowning.8
Recommendation-Specific Supportive Text
- There is no direct evidence evaluating PAD programs for cardiac arrest following drowning. Twenty-six observational studies and 1 RCT evaluating PAD programs in adult OHCA and 2 observational studies evaluating PAD programs in pediatric OHCA demonstrated improved outcomes.1,2,9,10 Two studies of PAD programs for cardiac arrest following drowning demonstrated feasibility in lifeboat and water park environments.5,11
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Adults and children in cardiac arrest due to electrical injury should receive standard resuscitation. |
Synopsis
Electricity causes various injuries depending on the voltage, duration of contact, type of current (alternating or direct), and pathway of current traveling through the body.1 Depending on the type of exposure, patients may experience significant burns, traumatic injuries, arrhythmias, cardiac injury, or cardiac arrest. Multiple arrhythmias are described following electrical injuries, including atrial fibrillation, VF, VT, and pulseless electrical activity (PEA).1,2 The care of patients with burns, cardiac dysfunction, myocardial infarction, and traumatic injuries from electricity is beyond the scope of this recommendation.
These recommendations inform the management of adults and children in cardiac arrest from electrocution.
Recommendation-Specific Supportive Text
- The mainstay of care of adults and children in cardiac arrest following electrical injury includes adherence to standard BLS and pediatric BLS as well as ALS and PALS guidelines, including rapid defibrillation and high-quality CPR as well as scene safety for responders. No clinical trials or controlled observational studies are available to inform the care of adults and children in cardiac arrest or peri-arrest following electrical injury. Numerous adult and pediatric case reports and case series describe patients with cardiac arrest, including with VF, VT, and PEA, who responded to standard resuscitation.2,3 There are adult and pediatric case reports of patients surviving with good neurologic outcome following prolonged CPR (up to 70 minutes) for cardiac arrest following electrical injury.4-6
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Adults and children with life-threatening gas embolism should receive 100% oxygen. |
| 2a | B-NR | 2. It is reasonable to use hyperbaric oxygen for adults with life-threatening gas embolism. |
| 2a | C-EO | 3. It is reasonable to use hyperbaric oxygen for children with life-threatening gas embolism. |
| 2a | C-EO | 4. It is reasonable to position adults and children with life-threatening gas embolism supine or in the recovery position, on a level surface. |
| 2b | C-EO | 5. For adults, it may be reasonable to attempt aspiration of life-threatening right ventricular gas embolism, using a needle or a catheter, when the location of gas is known and accessible. |
Synopsis
Arterial or venous gas embolism can occur due to medical intervention, trauma, barotrauma, decompression sickness, and other causes. Bubble composition may comprise air, nitrogen, and carbon dioxide.1
Gas in the arterial system rises to the least dependent vascular bed. Cerebral arterial gas embolism can occlude arteries, causing ischemic stroke. Coronary artery gas embolism can cause myocardial infarction, cardiogenic shock, or cardiac arrest. Venous gas can occlude right ventricular or pulmonary arterial blood flow, causing shock and cardiac arrest. Venous gas can reach the arterial system ("paradoxical gas embolism") through a patent foramen ovale or transpulmonary passage. In addition to obstructing blood vessels, gas embolism causes vascular injury, vasospasm, and thromboinflammation.2
Whether the same treatment is ideal for arterial and venous gas embolism, for all types of gas, or for all mechanisms of embolism production is unclear. No clinical trials have been conducted, and animal studies may not fully translate to humans. Treatment is informed by many case reports, case series, and observational comparison studies, almost all involving adults.
Nothing about the presence of gas embolism provides a contraindication to standard resuscitation, and some patients recover with standard resuscitation alone.3
The use of lidocaine or other medications for neuroprotection in patients with stroke due to cerebral arterial gas embolism is outside the scope of these guidelines.
These recommendations inform the management of adults and children with life-threatening gas embolism, including cardiac arrest.
Recommendation-Specific Supportive Text
- In addition to treating tissue hypoxia, supplemental oxygen increases the diffusion of nitrogen out of gas bubbles while also improving oxygenation of adjacent ischemic tissues.4 This recommendation is supported by adult and pediatric case reports.5
- and 3. Hyperbaric oxygen therapy for gas embolism is supported by 2 adult nonrandomized observational studies, adult case series, limited pediatric case series, and animal studies.3,6-10 Hyperbaric oxygen therapy compresses gas bubbles, increases diffusion of nitrogen and other gases from the bubble, oxygenates adjacent tissue, and reduces inflammatory cascades.11 It is not feasible to provide hyperbaric oxygen therapy for patients in cardiac arrest in most settings.
- The Undersea and Hyperbaric Medical Society recommends that people with gas embolism who have normal mental status be positioned supine and that those with altered mental status be positioned in the recovery position, which is consistent with standard first aid principles.12-14 The US Navy Diving Manual also recommends level positioning during transport to definitive therapy.15 Neither organization currently recommends head-down (Trendelenburg) positioning. These recommendations are not universally accepted, with some experts recommending the Durant position (left lateral decubitus, head down), at least for venous gas embolism.16,17 Studies of whether head-down positioning results in redistribution of intravascular bubbles are contradictory, and limited animal data suggest that head-down positioning may worsen cerebral edema.18-21 Effective CPR cannot be performed in the recovery (lateral decubitus) position, and standard resuscitation practice calls for compressions to be administered with the patient positioned supine (refer to "Part 7: Adult BLS" guidelines). Treatment recommendations from the Undersea and Hyperbaric Medical Society do not comment on age; supporting data are largely based on animal studies and adult case reports, and, thus, recommendations for children are extrapolated.
- Aspiration of gas from the right ventricle using either a needle or catheter is described in adult case reports and animal studies.22-24 The success rate of this procedure and whether it can safely be performed without ultrasound or fluoroscopic guidance is unknown.25 No data were identified for gas aspiration in children.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Chest compressions, bag-mask ventilation, defibrillation, suctioning, and endotracheal intubation should be considered aerosol-generating procedures (AGPs) for adults and children with confirmed or suspected high-consequence respiratory pathogens. |
| 1 | C-LD | 2. Resuscitation team members should wear PPE when in the resuscitation environment, including respiratory protection (N-95 respirator or purifying air-powered respirator), eye protection, gloves, and protection against contamination of clothing. |
| 1 | C-LD | 3. When performing endotracheal intubation of adults with confirmed or suspected high-consequence respiratory pathogens, video laryngoscopy is preferred compared with direct laryngoscopy. |
| 1 | C-EO | 4. When performing endotracheal intubation of children with confirmed or suspected high-consequence respiratory pathogens, video laryngoscopy is preferred compared with direct laryngoscopy. |
| 1 | C-EO | 5. Whenever possible, all ventilation devices applied to adults and children with confirmed or suspected high-consequence respiratory pathogens should include a high-efficiency particle air filter to remove fugitive aerosols from ventilation device outflow. |
| 1 | C-EO | 6. Resuscitation team leaders should minimize the number of people present in the room during resuscitation, consistent with effective resuscitation. |
| 2a | C-LD | 7. It is reasonable to dedicate a resuscitation team member to monitor and coach team members to adhere to PPE procedures. |
| 2a | C-LD | 8. It is reasonable to use mechanical CPR devices to provide compressions in adults with confirmed or suspected high-consequence respiratory pathogens, with the goal of maintaining consistent CPR quality while reducing the number of at-risk resuscitation team members. |
| 2a | C-EO | 9. It is reasonable to perform endotracheal intubation, rather than bag-mask ventilation, for adults with confirmed or suspected high-consequence respiratory pathogen to reduce the number of respirable particles generated during resuscitation. |
| 2b | C-EO | 10. It may be reasonable to perform endotracheal intubation, rather than bag-mask ventilation, for children with confirmed or suspected high-consequence respiratory pathogens to reduce the number of respirable particles generated during resuscitation. |
| 3: No Benefit | C-LD | 11. The use of intubating boxes and other improvised barrier devices during intubation of adults and children with confirmed or suspected high-consequence respiratory pathogens is not recommended. |
Synopsis
Worldwide pandemics caused by high-consequence pathogens, as termed by the Centers for Disease Control, are common. Recent examples include SARS (2002–2003), influenza H1N1 (2009–2010), Middle East respiratory syndrome coronavirus (MERS-CoV, approximately 2012–2015), and SARS-CoV-2 (or COVID-19, approximately 2019–2022). These infections are spread by direct contact with a combination of respiratory secretions, droplets, and aerosols.1 Effective vaccines and treatment may not be available during the early stages of a pandemic, putting resuscitation team members at risk. The AHA released interim guidance for BLS and ALS modifications for patients with suspected or confirmed SARS-CoV-2 in 2020 and 2022 to provide effective resuscitation while minimizing risk.2,3 The recommendations in this section supersede that guidance, broaden the recommendations to encompass all high-consequence pathogens, and acknowledge the limitations of not knowing the transmissibility of future pathogens.
Most studies in this area relate to IHCA, with less literature related to emergency medical services personnel and almost no studies related to lay rescuers. Lay rescuer CPR rates decreased in some regions during the early stages of the SARS-CoV-2 pandemic, and resuscitation outcomes from OHCA were worse compared with before the pandemic.4-8 Availability of PPE may improve laypeople’s willingness to perform CPR.9 The most effective strategy to protect members of the general public providing CPR during a disease outbreak represents a major knowledge gap.
Ethical considerations of performing resuscitation in the setting of personal risk are discussed in “Part 3: Ethics.”
Recommendation-Specific Supportive Text
1. Systematic reviews identified CPR, bag-mask ventilation, intubation, and suctioning as AGPs.10-16 Other studies were less conclusive about CPR, intubation, and suctioning as AGPs.10,17-20 A human and animal study that evaluated aerosol particle concentration during cardiac arrest found that compressions, bag-mask ventilation, and defibrillation all generate aerosols.21
2. The use of PPE, including respiratory protection, eye protection, gloves, and barrier gowns reduces the risk of infection to health care personnel.10,13-15,17 In an observational study of over 1500 health care professionals wearing PPE for approximately 180 SARS-CoV-2–positive patients requiring AGPs, only 1 health care professional became positive in the 2 weeks after the encounter.22,23 For prehospital professionals wearing PPE, only 1% became SARS-CoV-2 positive following care for approximately 190 patients with SARS-CoV-2 receiving AGP.24 Simulation studies show a delay in CPR initiation of 30 to 60 seconds to don PPE.11,25-27 Simulation studies about the effect of PPE on CPR quality are mixed.15,28-45 An IHCA study of 800 adult resuscitations before and after implementation of a resuscitation PPE policy showed a delay to initiation of compressions by 20 seconds, 2 minutes for rhythm analysis, no difference to first shock, 1 minute for epinephrine, and 1 minute 40 seconds for intubation.46 A prospective observational study found similar first-pass intubation success when intubation (98% adults) was performed by a health care professional wearing full PPE.47 No difference in survival was found in this study or in 2 other studies of adult OHCA and emergency department resuscitation.48,49 In a prospective observational study of 3435 adult emergency department intubations, rates of peri-intubation adverse events were not increased when full barrier PPE was used.22
3. and 4. Simulation studies and adult observational studies of intubations with PPE use suggest that operators wearing PPE could perform video laryngoscopy faster than direct laryngoscopy with similar intubation success rates and that video laryngoscopy could be performed with the intubator’s face farther from the source of aerosols.50-53 One adult observational study showed increased time to intubation with video laryngoscopy compared with direct laryngoscopy.50 A large RCT of adult intubation conducted near the end of the SARS-CoV-2 pandemic (March to November 2022) found better first-attempt intubation success with video laryngoscopy than direct laryngoscopy.54 The type of PPE used was not reported in this study. No pediatric data on the use of video versus direct laryngoscopy with PPE were identified, thus, the recommendation for children is extrapolated from the adult data.
5. High-efficiency particle air and high-efficiency bacterial-viral filters remove more than 99.9% of transmissible viruses and other infectious aerosols from prefiltered air. Placing a filter with high viral filtration efficacy on the exhaust limb of ventilation devices, such as bag-mask ventilation devices or bag devices attached to a tracheal tube or supraglottic airway, reduces fugitive aerosol emissions in the treatment environment and is, therefore, recommended by anesthesiology safety groups and other organizations.55-62
6. Decreasing the number of people in the room during resuscitation reduces the number of people at risk of disease transmission. A simulated study showed that although quality of CPR metrics did not change with less personnel in the room, there was a delay in resuscitation task completion with less personnel in the room.25 An audio link to team members outside the room enables tasks, such as contemporaneous clinical documentation and drug preparation, to be performed by personnel outside the high-risk environment.
7. Resuscitation team adherence to proper PPE procedures is often incomplete, and proper mask fit during compressions can be compromised.11,22,26,63,64 Just as assigning a team member to monitor and provide real-time feedback about CPR improves CPR quality, dedicating a team member to supervise PPE use may improve PPE adherence and, therefore, team member safety. Ideally, this would be done without exposing an additional person to the infectious resuscitation environment. It is standard in cases of suspected viral hemorrhagic fever to have a “trained observer” who is dedicated to providing real-time feedback to team members about their PPE use.65,66 This concept has been tested in simulated SARS-CoV-2 resuscitation and using remote video monitoring.26,67-69
8. Performing chest compressions while wearing PPE increases rescuer fatigue, and several simulation studies showed that wearing PPE also negatively impacts compression depth and resuscitation timing.15,29,30,36,41-45,70-73 Mechanical CPR devices can maintain high-quality chest compressions while reducing the number of people required to perform resuscitation. Although a simulation study found that mechanical CPR devices are associated with better compression quality than human compressors wearing PPE, an observational study of adults with OHCA (421 propensity-matched pairs) did not find a difference in survival or neurologic outcomes when a mechanical CPR device was used compared with manual CPR with PPE.74,75 No data were identified on mechanical CPR devices compared with manual CPR with PPE in children.
9. and 10. A case report of a healthy adult volunteer who had an awake intubation showed that the risk of exposure to aerosol particles is increased for personnel within 3 meters of the patient.76 However, a study of 3 adults undergoing intubation showed that the mean particle concentrations were 200 to 300 times baseline during bag-mask ventilation, compared with 30 to 50 times baseline during endotracheal tube insertion.77 While no longer emphasized in standard resuscitation, endotracheal intubation redirects a patient’s exhaled gas, respiratory droplets, and respiratory aerosols away from personnel in the room and contains them.76,77 No pediatric data on endotracheal intubation to contain respiratory droplets and aerosol particles were identified, thus, the recommendation for children is extrapolated from the adult data. No studies were identified on the use of supraglottic airways to contain respiratory droplets and aerosol particles.
11. Intubation boxes, also known as aerosol boxes, were evaluated in a systematic review that included 20 simulation studies and showed a reduction in droplets but not aerosols and an increased time to endotracheal tube placement.78 This was further supported by more recent simulation studies with the same conclusions, although interpretation of the data could be limited by box design heterogeneity between studies.79-83 Minimizing gaps and using negative pressure devices may improve the box’s performance. Patient draping has also been investigated with similar issues.28,40,79,83,84
Table 2 summarizes the key steps to reduce transmission of these pathogens.
Table 2. Transmission Reduction in High-Consequence Respiratory Pathogens in Cardiac Arrest
| Key steps to take to reduce the risk of transmission of high-consequence respiratory pathogens during treatment of cardiac arrest in adults and children |
|---|
|
|
– N95 respirator or PAPR, eye protection, gloves, gown
|
|
– Consider designating a PPE monitor or coach |
|
|
|
– Monitor for team member fatigue |
|
|
– Video laryngoscopy is preferred |
|
– Use a HEPA filter to reduce fugitive aerosols from bag-mask ventilation device or ventilator |
|
– Intubating boxes and other improvised barrier devices are not recommended |
CPR indicates cardiopulmonary resuscitation; HEPA, high-efficiency particle air; PAPR, powered air-purifying respirator; and PPE, personal protective equipment.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. The effectiveness of IV calcium administration for adults and children in cardiac arrest from suspected hyperkalemia is not well established. |
| 2b | C-EO | 2. The effectiveness of IV sodium bicarbonate administration for adults and children in cardiac arrest from suspected hyperkalemia is not well established. |
| 2b | C-EO | 3. The effectiveness of IV insulin and glucose for adults and children in cardiac arrest from suspected hyperkalemia is not well established. |
| 3: No Benefit | C-EO | 4. Inhaled β2-agonists for adults and children in cardiac arrest from suspected hyperkalemia is not recommended. |
Synopsis
Hyperkalemia, defined as serum potassium concentration greater than 5.0 mEq/L in adults and greater than 5.5 mEq/L in children, can lead to life-threatening cardiac arrhythmias and cardiac arrest.1,2 While the prevalence of hyperkalemia in the adult population is estimated at 2% to 3%, hospitalized adults face a greater incidence of severe hyperkalemia (greater than 6.5 mEq/L), ranging from 1% to 10%.3-6 Risk factors contributing to the development of hyperkalemia in both adults and children include impaired kidney function, potassium supplements, potassium-sparing diuretics, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ARBs), brisk hemolysis, and crush injuries. Acidemia during cardiac arrest can also temporarily cause potassium to shift extracellularly, resulting in elevated serum potassium concentrations, which may conflate the etiology of cardiac arrest.7 Laboratory confirmation is not required for initial treatment, although patient history and electrocardiogram changes can support suspicion of hyperkalemia as an underlying etiology. Characteristic electrocardiogram abnormalities of hyperkalemia may include peaked T waves, flattened P wave, PR interval lengthening, QRS complex prolongation, and ST segment depression followed by merging of S and T waves (sine wave), though the sensitivity and specificity of these findings are low.8
These recommendations are only applicable to the management of adults and children in cardiac arrest from hyperkalemia and do not discuss the treatment of life-threatening hyperkalemia with therapies such as loop diuretics and potassium binders.
Recommendation-Specific Supportive Text
- The benefits of IV calcium administration for myocardial protection during hyperkalemia in the setting of cardiac arrest are based on an 1883 in vitro study, a 1939 dog study, and a series of 5 adult cases in 1964.9-11 Calcium administration alone in a randomized controlled porcine model of hyperkalemic cardiac arrest did not increase ROSC (OR, 1.2; 95% CI, 0.4–4.0; PP=0.23) when compared with placebo.12 There are no high-quality human observational studies or clinical trials evaluating IV calcium to treat patients with hyperkalemic cardiac arrest. One human adult retrospective observational study on the effectiveness of calcium and sodium bicarbonate is limited by small sample size, retrospective design, and multiple conflicting regression analyses, leading to low certainty of results.13 The study did not inform this recommendation. Furthermore, no significant benefit of IV calcium was observed in a subanalysis of adult out-of-hospital PEA arrests in which hyperkalemia was suspected as defined by electrocardiogram abnormalities rather than prearrest potassium concentrations.14 Limited data in children seem to demonstrate findings consistent with adults. In a separate subgroup analysis of observational data in pediatric hospitals, 8 children receiving calcium during in-hospital hyperkalemia-induced cardiac arrest showed no significant differences or trends in survival to hospital discharge or favorable neurological outcome.15
- Sodium bicarbonate shifts potassium intracellularly and is theoretically beneficial when severe hyperkalemia exists with cardiac arrest. In a randomized controlled pig model of hyperkalemic cardiac arrest, sodium bicarbonate significantly increased ROSC (24/26 [92%] versus 13/26 [50%]; OR, 12.0; 95% CI, 2.3–61.5; P = 0.003) and reduced time to ROSC (HR, 3.6; 95% CI, 1.8–7.5; P less than 0.001).12 One human adult retrospective observational study on the effectiveness of calcium and sodium bicarbonate is limited by small sample size, retrospective design, and multiple conflicting regression analyses, leading to low certainty of results.13 The study did not inform this recommendation. There is a paucity of published literature of sodium bicarbonate in children, and, thus, recommendations are extrapolated from available adult data.
- Insulin and glucose shift potassium intracellularly. Treatment with IV insulin in the setting of hyperkalemic cardiac arrest has produced variable results and is limited to case reports and case series. Notably, standard IV insulin therapy was ineffective in a reported case series of 11 adults and 5 children.16 However, a recent case report of 2 adults indicated a potential benefit of high-dose insulin (1 U/kg IV) on ROSC in the presence of hyperkalemia.17 The ILCOR 2024 International Consensus on CPR and Emergency Cardiovascular Care Science With Treatment Recommendations recommends the combination of insulin and glucose for adults and children with cardiac arrest from suspected hyperkalemia based on indirect non–cardiac arrest evidence demonstrating the lowering of serum potassium concentrations.18,19 The use of insulin and glucose risks hypoglycemia and has the opportunity cost of forgoing other potentially efficacious interventions. Doses for insulin and glucose for cardiac arrest from suspected hyperkalemia are listed in Table 3.
- Stimulation of the β2-adrenergic receptors promotes intracellular movement of potassium by activation of the Na-K ATPase pump. There are no data demonstrating additional benefit using additional β2-adrenergic agonists over basic resuscitation in either adults or children with cardiac arrest from suspected hyperkalemia. Epinephrine is far more effective at β-adrenergic activation than β2-specific agonists (eg, albuterol or salbutamol).20 Furthermore, differential effects on serum potassium concentrations may occur over the course of therapy.21 Excessive β2-agonist–induced peripheral vasodilation and the potential for interruptions or ineffective CPR to deliver inhaled therapy could negatively impact patient outcomes.
Table 3. Insulin-Glucose Dosing for Cardiac Arrest From Suspected Hyperkalemia
| Population | Glucose | Insulin | Administration notes |
|---|---|---|---|
|
Adults |
|||
|
Standard dose |
D50W or glucose 50%: 50 g IV bolus |
10 Units regular insulin |
Administer insulin IV over 15–30 minutes |
|
Children |
|||
|
Standard dose |
D10W or Glucose 10%: 5 mL/kg IV |
0.1 Units/kg IV bolus regular insulin |
Administer insulin IV over 15–30 minutes |
D50W indicates 50% dextrose in water; D10W, 10% dextrose in water; and IV, intravenous.
Life-threatening hyperthermia (defined as a core temperature greater than 40 °C [104 °F]) is a preventable cause of cardiac arrest that has multiple etiologies, including exertional heatstroke, environmental exposure, and medication or drug overdose. Animal data suggests that temperature and duration are critical determinants of survival.1-3 Prolonged temperature elevation leads to multiorgan failure that resembles septic shock.1
Children are at higher risk of hyperthermia compared to adults for several reasons. Children are at higher risk for heat gain because they have larger ratios of body surface area to body mass, increased metabolic heat production, and ineffective sweat glands to dissipate heat. Young children also are unable to recognize or self-extricate from hyperthermic environments.4,5
No RCT on cooling patients with hyperthermic cardiac arrest has been performed. As a result, recommendations are made from data derived from adults with life-threatening hyperthermia and experimental models of hyperthermia in human volunteers. The following recommendations only apply to adults and children with exertional heatstroke, environmental exposure, and medication or drug overdose. The treatment of patients with malignant hyperthermia and sepsis is outside the scope of these recommendations.
Specific discussion of the management of adults and children with life-threatening cocaine and sympathomimetics poisoning can be found elsewhere (refer to the Toxicology: Cocaine and Toxicology: Sympathomimetics sections). These recommendations inform the management of adults and children with life-threatening hyperthermia, including cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Active cooling is recommended to be initiated concurrently with standard resuscitation for adults with life-threatening hyperthermia. |
| 2a | B-R | 2. It is reasonable to choose immersion in ice water (1–5 °C [33.8–41 °F]) over other cooling methods in adults with life-threatening hyperthermia. |
| 2a | B-R | 3. If ice water immersion is not available, it is reasonable to choose immersion in tepid water (8–17 °C [46.4–62.6 °F]) or tarp-assisted ice water cooling over other cooling methods in adults with life-threatening hyperthermia. |
| 2a | C-LD | 4. It is reasonable to cool adults with life-threatening hyperthermia as rapidly as possible with a decrease of at least 0.15 °C/min (0.27 °F/min). |
| 2a | C-LD | 5. It is reasonable to stop active cooling for life-threatening hyperthermia when the core temperature of the adult patient reaches 38.6 °C (101.5 °F). |
| 3: No Benefit | B-R | 6. Dantrolene is not useful to treat adults with life-threatening hyperthermia from causes other than malignant hyperthermia. |
Recommendation-Specific Supportive Text
- Standard resuscitation enhances circulation, which helps dissipate excess heat through vascular distribution of blood from the core to the extremities.6 Rapid body cooling addresses hyperthermia while preventing further rises in temperature.6
- A systematic review of cooling rates that included 63 studies of primarily adults (25 randomized clinical trials, 12 nonrandomized clinical trials, 2 cohort studies, and 24 case series) concluded that ice water immersion provided faster cooling rates than numerous other techniques (Figure 2).7 The superiority of ice water (1–5 °C [33.8–41 °F]) immersion is supported by 1 adult volunteer RCT.8 This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
- When ice water immersion is not available, full body immersion in tepid water (8–17 °C [46.4–62.6 °F]) still often achieves adequate cooling rates in multiple systematic reviews of primarily adult populations.7,10 When immersion is not feasible, tarp-assisted ice water cooling produces adequate cooling rates in adults (Figure 2).10,11 This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
- A systematic review of case reports and case series identified an increase in mortality in adults cooled slower than 0.15 °C/min (0.27 °F/min).12 This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
- A controlled trial of 10 healthy adult volunteers with non–life-threatening hyperthermia (core temperature, 39.5 °C [103.1 °F]) demonstrated that those who were removed from ice water immersion at a core temperature of 38.6 °C (101.5 °F) had lower risk of overcooling compared with those removed at 37.5 °C (99.5 °F).13 Overcooling to below normothermia could cause complications, such as coagulopathy and adverse hemodynamic effects.14
- Four studies that investigated the effects of dantrolene in life-threatening hyperthermia (from causes other than malignant hyperthermia) were included in the data review and demonstrated mixed results. An adult case series (n=20) of dantrolene use in hyperthermia for exposure-related heat stroke was associated with more rapid cooling but no difference in neurologic outcomes.15 An RCT (n=52 adults) of dantrolene (2 mg/kg IV) for environmental hyperthermia (greater than 40.1 °C [104.2 °F]) did not find a reduction in cooling time.16 Data on dantrolene for 3,4-methylenedioxymethamphetamine (MDMA)–induced hyperthermia is also conflicting. A meta-analysis of patients aged ≥15 years identified case reports and case series totaling 71 cases and found that dantrolene use was associated with improved survival.17 However, a case series of 9 adults did not find clinical differences between patients who received dantrolene versus those who did not.18 This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Active cooling is recommended to be initiated concurrently with standard resuscitation for children with life-threatening hyperthermia. |
| 2a | C-EO | 2. It is reasonable to choose immersion in ice water (1–5 °C; 33.8–41 °F) over other cooling methods in children with life-threatening hyperthermia. |
| 2a | C-EO | 3. If ice water immersion is not available, it is reasonable to choose immersion in tepid water (8–17 °C; 46.4–62.6 °F) or tarp-assisted ice water cooling over other cooling methods in children with life-threatening hyperthermia. |
| 2a | C-EO | 4. It is reasonable to cool children with life-threatening hyperthermia as rapidly as possible with a decrease of at least 0.15 °C/min (0.27 °F/min). |
| 2a | C-EO | 5. It is reasonable to stop active cooling for life-threatening hyperthermia when the core temperature of a child reaches 38.6 °C (101.5 °F). |
| 3: No Benefit | C-EO | 6. Dantrolene is not useful to treat children with life-threatening hyperthermia from causes other than malignant hyperthermia. |
Recommendation-Specific Supportive Text
- Standard resuscitation enhances circulation, which helps dissipate excess heat through vascular distribution of blood from the core to the extremities.6 Rapid body cooling addresses hyperthermia while preventing further rises in temperature.6
- A systematic review of cooling rates that included 63 studies of primarily adults (25 randomized clinical trials, 12 nonrandomized clinical trials, 2 cohort studies, and 24 cases series) concluded that ice water immersion provided faster cooling rates than numerous other techniques (Figure 2).7 The superiority of ice water (1–5 °C [33.8–41 °F]) immersion is supported by 1 adult volunteer RCT.8 No pediatric data for cooling rates with ice water immersion were identified, and, thus, the recommendation for children is extrapolated from this adult data. This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
- When ice water immersion is not available, full body immersion in tepid water (8–17 °C [46.4–62.6 °F]) still often achieves adequate cooling rates, as shown in multiple systematic reviews of primarily adult populations.7,10 When immersion is not feasible, tarp-assisted ice water cooling produces adequate cooling rates in adults (Figure 2).10,11 No pediatric data for cooling rates with tepid water immersion or tarp-assisted ice water cooling procedures were identified, and, thus, the recommendation for children is extrapolated from this adult data. This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
- A systematic review of case reports and case series identified an increase in mortality in adults cooled slower than 0.15 °C/min (0.27 °F/min).12 No pediatric data for cooling rates were identified, and, thus, the recommendation for children is extrapolated from this adult data. This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9
- A controlled trial of 10 healthy adult volunteers with non–life-threatening hyperthermia (core temperature, 39.5 °C [103.1 °F]) demonstrated that those who were removed from ice water immersion at a core temperature of 38.6 °C (101.5 °F) had lower risk of overcooling compared with those removed at 37.5 °C (99.5 °F).13 Overcooling to below normothermia could cause complications, such as coagulopathy and adverse hemodynamic effects.14 No pediatric data for the optimal temperature at which to terminate cooling were identified, and, thus, the recommendation for children is extrapolated from this adult data.
- Four studies that investigated the effects of dantrolene in life-threatening hyperthermia (from causes other than malignant hyperthermia) were included in the data review and demonstrated mixed results. An adult case series (n=20) of dantrolene use in hyperthermia for exposure-related heat stroke was associated with more rapid cooling but no difference in neurologic outcomes.15 An RCT (n=52 adults) of dantrolene (2 mg/kg IV) for environmental hyperthermia (greater than 40.1 °C [104.2 °F]) did not find a reduction in cooling time.16 Data on dantrolene for MDMA-induced hyperthermia are also conflicting. A meta-analysis of patients aged ≥15 years identified case reports and case series totaling 71 cases and found that dantrolene use was associated with improved survival.17 Both pediatric patients (a 16- and a 17-year-old patient) included in the study died.17 Furthermore, a case series in adults did not find clinical differences between patients who received dantrolene versus those who did not.18 Limited pediatric data on the use of dantrolene to treat life-threatening hyperthermia were identified; thus, the recommendation for children is extrapolated primarily from the adult data. This recommendation is consistent with the 2025 Society of Critical Care Medicine Guidelines for the Treatment of Heat Stroke.9

Reproduced from Douma MJ, Aves T, Allan KS, et al. First aid cooling techniques for heat stroke and exertional hyperthermia: A systematic review and meta-analysis. Resuscitation. 2020;148:173-190. doi: 10.1016/j.resuscitation.2020.01.007
Severe environmental hypothermia (core body temperature, less than 30 °C [86 °F]) can cause cardiac arrest as well as findings that mimic death.1 Pulse and respiratory rates may be slow or difficult to detect, and the electrocardiogram may show asystole. Apparent rigor mortis or fixed dilated pupils may not be reliable signs of death in this setting.2-4 Hypothermia decreases the body’s metabolic rate and oxygen consumption, improving the chance of survival and neurological recovery even after prolonged cardiac arrest.5-7
Hypothermic cardiac arrest often occurs in dangerous environmental conditions, and verification of scene safety prior to implementing resuscitative measures is important. Following removal from environmental exposure and removal of wet clothing, passive rewarming may include techniques such as providing a blanket and active rewarming may include providing a warm forced-air blanket, administration of warmed IV or IO fluids, warm humidified oxygen, warm water lavage of the thoracic and peritoneal cavities, blood rewarming using a kidney replacement therapy circuit, or use of endovascular temperature control catheters.8-13 Warming through ECLS is possible at some centers and also provides circulatory support.
Children are at higher risk of hypothermia compared with adults due to a larger ratio of body surface area to body mass and decreased insulation by fat. Infants cannot produce heat through shivering, and both infants and children have limited glycogen stores required to produce heat and are unable to self-extricate from hypothermic environments.14
In the absence of human evidence, these guidelines do not make a recommendation about the use of antiarrhythmic medications for patients in cardiac arrest from environmental hypothermia. These recommendations are specific to hypothermia management. Other specific treatment recommendations related to the setting of drowning can be found elsewhere (refer to Drowning).15 Treatment recommendations related to avalanche are outside of the scope of these guidelines.
These recommendations inform the management of adults and children with life-threatening environmental hypothermia, including cardiac arrest. Other specific treatment recommendations related to adults and children with hypothermia that is not immediately life-threatening can be found in the AHA and American Red Cross 2024 Guidelines for First Aid.16
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. For adults with life-threatening environmental hypothermia with no obvious lethal injury, full resuscitative measures should be performed immediately and continued concurrently with rewarming. |
| 2a | B-NR | 2. It is reasonable to rewarm adults in hypothermic cardiac arrest using ECLS. |
| 2a | B-NR | 3. It is reasonable to use prognostication scores to guide the decision for initiating ECLS rewarming for adults in hypothermic cardiac arrest. |
| 2b | C-LD | 4. It may be reasonable to rewarm adults with severe environmental hypothermia (core temperature less than 28 °C [82.4 °F]) and not in cardiac arrest using ECLS. |
| 2b | C-LD | 5. For adults in hypothermic cardiac arrest, it may be reasonable to defibrillate once and if unsuccessful, defer further defibrillation until the core temperature is ≥30 °C (86 °F). |
| 2b | C-LD | 6. It may be reasonable to rewarm adults with life-threatening environmental hypothermia with ECLS at a rate of 1.5–5 °C/hour (2.7–9 °F/hour). |
| 2b | C-EO | 7. It may be reasonable to defer administration of epinephrine until the core temperature is ≥30 °C (86 °F) for adults in hypothermic cardiac arrest. |
| 2b | C-EO | 8. If standard CPR is not possible due to environmental conditions, it may be reasonable to perform delayed or intermittent CPR during the resuscitation of adults in hypothermic cardiac arrest to facilitate rapid extraction. |
| 2b | C-EO | 9. It may be reasonable to transport adults in hypothermic cardiac arrest to a center with ECLS capability if it can be reached within 6 hours. |
| 3: No Benefit | B-NR | 10. It is not recommended to use end-tidal carbon dioxide (ETCO2) to predict outcomes for adults in hypothermic cardiac arrest. |
Recommendation-Specific Supportive Text
- Obvious lethal injury (eg, decapitation, truncal transection, whole body incineration, or frozen solid) are suggested conditions to withhold or terminate CPR.17 Absent these signs, numerous case reports and the International Hypothermia Registry describe ROSC prior to or concurrently with rewarming in adults.18-20
- A multicenter prospective observational study of 242 adults with severe environmental hypothermia showed improved survival and favorable neurologic outcome for adults in cardiac arrest treated with ECMO compared with conventional rewarming.21 Multiple retrospective observational studies of adults in hypothermic cardiac arrest rewarmed with ECLS have demonstrated a survival benefit and improved neurological outcome over conventional CPR.22-24 The use of ECMO is preferable over CPB; a meta-analysis including 464 adults and children in cardiac arrest due to hypothermia showed a 41% greater probability of survival with ECMO.25
- The Hypothermia Outcome Prediction After ECLS (HOPE) probability score includes age, sex, asphyxia, CPR duration, serum potassium concentration, and temperature.26 A HOPE score less than 0.1 has been validated for predicting mortality of adults in hypothermic cardiac arrest placed on ECLS for rewarming.27,28 The HOPE score is superior to serum potassium threshold of ≥12 mmol/L alone in adults and children.26 The International Accidental Hypothermia ECLS (ICE) survival score includes sex, asphyxia, and serum potassium concentration, and a score greater than 12 has also been validated in predicting survival after hypothermic cardiac arrest in adults and children.29 Other prognostic factors in isolation have been associated with higher mortality in adults and children with hypothermic cardiac arrest, including high serum potassium concentration, high serum lactate, low pH, asphyxia, unwitnessed cardiac arrest, male sex, older age, higher or lower initial body temperature, and longer resuscitation duration.19,20,25,27,30-33 These prognostic factors are less well-validated than the HOPE or ICE scores. The ELSO eligibility criteria for ECLS in normothermic OHCA may miss adults in hypothermic cardiac arrest who would benefit from ECLS.11
- Retrospective observational studies in adults with core temperature less than 28 °C (82.4 °F) who are not in cardiac arrest have shown successful and even faster rewarming with ECLS compared with conventional methods.22,24,34,35 A multicenter prospective observational study showed improved survival and neurological outcomes with the use of ECMO for adults in hypothermic cardiac arrest. However, adults with hemodynamic instability (eg, not in cardiac arrest) from hypothermia who were placed on ECMO had decreased event-free days (ICU, ventilator, and catecholamine administration) and increased hemorrhagic complications without the benefit of improved survival or neurological outcome.21
- Defibrillation before rewarming can be successful in adults, but the hypothermic heart may be less responsive to defibrillation.18,20,32,36,37 The temperature at which defibrillation should first be tried and the number of defibrillation attempts that should be made are not well-studied in humans. If VT or VF persists, excessive defibrillation attempts may lead to myocardial injury. In a large retrospective observational study, while a shockable initial rhythm in adults in hypothermic OHCA was associated with better survival and neurologically favorable survival, prehospital defibrillation and increasing the number of defibrillation attempts were not.38 However, only a small number of adults with shockable rhythms were not defibrillated, so the study may have been underpowered to detect a difference in outcomes in this population. The Wilderness Medical Society guideline recommends defibrillating once initially and deferring additional defibrillation attempts until core temperature reaches 30 °C (86 °F) when defibrillations are more likely to be successful.39,40 ERC guidelines allow up to 3 defibrillation attempts before rewarming to 30 °C (86 °F).41
- The optimal rate for rewarming hypothermic adults with ECLS is unknown. Slow rewarming may delay the return of organ perfusion, and rapid rewarming may cause relative cerebral hyperthermia, potentially worsening neurological outcomes. A secondary review of a meta-analysis of observational studies and case reports of 658 adults and children rewarmed with ECLS evaluated the optimal threshold rewarming rate. Rewarming rates less than 5 °C/hour (9 °F/hour) had a 2.41 greater odds (95% CI, 1.75–3.31) of neurologically intact survival over rewarming rates greater than 5 °C/hour (9 °F/hour).42 Neurologically intact survival decreased by 1.9% for each 1 °C/hour (1.8 °F/hour) increase in rewarming rate.42 Another meta-analysis of case reports and case series showed a lower relative hazard of death in adults who were rewarmed at rates between 1.5 °C (2.7 °F) and 4 °C/hour (7.2 °F), though the former study had a substantially higher mean rewarming rate than the latter.30
- The temperature at which epinephrine should first be administered and the optimal dosing interval in the severely hypothermic adult has not been well-studied. Some adult and adolescent cases of hypothermic cardiac arrest have normal responses to epinephrine before rewarming.18 However, hypothermic hearts may be less responsive to cardiovascular drugs, and slowed metabolism due to hypothermia raises concerns for epinephrine accumulation and toxicity.41,43,44
- In cases of prolonged CPR or dangerous environmental conditions, it may be suboptimal or impossible to perform continuous high-quality CPR. Delayed or interrupted CPR may facilitate rapid extraction or transportation and allow for optimal CPR as soon as feasible or safe. Successful resuscitation has been documented in adult cases with delayed or intermittent CPR.45 The acceptable frequency and duration of CPR interruptions and which adults can best tolerate this are unknown.
- Rapid hospital transport is important to allow for aggressive rewarming with both noninvasive and invasive techniques. Two studies utilizing the same database conflicted on the association of low-flow time (time from cardiac arrest to the establishment of ECMO) with outcomes for adults in cardiac arrest due to hypothermia.46,47 An observational study showed that transportation to centers with ECLS capability improved neurologically favorable outcomes and 1-month survival in adults.38 Adults have been resuscitated using ECLS after hypothermic cardiac arrest lasting over 6 hours, so the ERC supports transporting to an ECLS-capable center if within 6 hours.41,48,49
- ETCO2 measurement by waveform capnography is utilized for confirmation of correct endotracheal tube placement and monitoring ventilation during endotracheal intubation. For adults in nonhypothermic cardiac arrest, ETCO2 is often used to monitor and optimize CPR quality, and an abrupt increase in ETCO2 can indicate ROSC. In an observational study comparing adults with and without hypothermic cardiac arrest assessed for ECLS, those in hypothermic cardiac arrest had significantly lower ETCO2 and temperature was positively correlated with ETCO2.27 Also, 45% of adult survivors had ETCO2 ≤10 mm Hg at hospital admission and ETCO2 value did not predict survival.27
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For children with life-threatening environmental hypothermia with no obvious lethal injury, full resuscitative measures should be performed immediately and continued concurrently with rewarming. |
| 2a | C-LD | 2. It is reasonable to rewarm children in hypothermic cardiac arrest using ECLS. |
| 2a | C-LD | 3. It is reasonable to use prognostication scores to guide the decision for initiating ECLS rewarming for children in hypothermic cardiac arrest. |
| 2b | C-LD | 4. It may be reasonable to rewarm children with life-threatening environmental hypothermia with ECLS at a rate less than 5 °C/hour (9 °F/hour). |
| 2b | C-EO | 5. It may be reasonable to rewarm children with environmental hypothermia (core temperature less than 28 °C [82.4 °F]) and not in cardiac arrest using ECLS. |
| 2b | C-EO | 6. For children with hypothermic cardiac arrest, it may be reasonable to defibrillate once and if unsuccessful, defer further defibrillation until the core temperature is ≥30 °C (86 °F). |
| 2b | C-EO | 7. It may be reasonable to defer administration of epinephrine until core temperature is ≥30 °C (86 °F) for children in hypothermic cardiac arrest. |
| 2b | C-EO | 8. If standard CPR is not possible due to environmental conditions, it may be reasonable to perform delayed or intermittent CPR during the resuscitation of children in hypothermic cardiac arrest to facilitate rapid extraction. |
| 2b | C-EO | 9. It may be reasonable to transport children in hypothermic cardiac arrest to a center with ECLS capability if it can be reached within 6 hours. |
| 3: No Benefit | B-NR | 10. It is not recommended to use ETCO2 to predict outcomes for children in hypothermic cardiac arrest. |
Recommendation-Specific Supportive Text
- Obvious lethal injury (eg, decapitation, truncal transection, whole body incineration, or frozen solid) are suggested conditions to withhold or terminate CPR.17 Absent these signs, numerous case reports and the International Hypothermia Registry describe ROSC prior to or concurrently with rewarming in adults.18-20 Data for chest compressions for children in cardiac arrest from environmental hypothermia is lacking, and the recommendation is extrapolated from adult data.
- A multicenter prospective observational study and multiple retrospective observational studies in adults have shown a survival benefit and favorable neurological outcomes with ECLS over conventional CPR for adults in cardiac arrest due to severe environmental hypothermia over conventional CPR.21-24 In a case series of children with hypothermic drowning, survival was better with conventional rewarming compared with ECMO.50 However, due to clear clinical differences in illness severity with the ECMO group having lower pH, higher serum potassium, and longer submersion time, this case series does not change the recommendation for use of ECLS in children. ECMO is preferable over CPB; a meta-analysis including 464 adults and children in cardiac arrest due to hypothermia showed a 41% greater probability of survival with ECMO.25
- The HOPE probability score includes age, sex, asphyxia, CPR duration, serum potassium concentration, and temperature and has been validated in a cohort of adults and children for predicting mortality and ineffective use of extracorporeal rewarming.27,28 In adults, a threshold HOPE score of less than 0.1 has been validated, but there are cases of children surviving with lower HOPE scores.28,51 The HOPE score may be useful in prognostication for children with a more conservative lower cutoff of 0.05.51 The ICE survival score includes sex, asphyxia, and serum potassium concentration, and a score greater than 12 has also been validated in predicting survival after hypothermic cardiac arrest in a mixed cohort of adults and children.29 Other prognostic factors in isolation have been associated with higher mortality in combined cohorts of adults and children in hypothermic cardiac arrest, including high serum potassium concentration, high serum lactate, low pH, asphyxia, unwitnessed cardiac arrest, male sex, older age, greater initial body temperature, and longer resuscitation duration.19,25,27,33 These prognostic factors are less well-validated than the HOPE or ICE scores. The HOPE score is superior to serum potassium threshold of ≥12 mmol/L alone in predicting survival in a cohort of adults and children.26
- The optimal rate for rewarming hypothermic children with ECLS is unknown. Slow rewarming may delay the return of organ perfusion, and rapid rewarming may cause relative cerebral hyperthermia, potentially worsening neurological outcomes. An analysis of case report data from 658 adults and children aggregated in the International Accidental Hypothermia Extracorporeal Life Support dataset evaluated the optimal threshold rewarming rate. Rewarming rates less than 5 °C/hour (9 °F/hour) had a 2.41 greater odds (95% CI, 1.75–3.31) of neurologically intact survival over rewarming rates greater than 5 °C/hour (9 °F/hour).42 Neurologically intact survival decreased by 1.9% for each 1 °C/hour (1.8 °F/hour) increase in rewarming rate.42
- There are no studies on rewarming with ECLS in children with severe environmental hypothermia and not in cardiac arrest, so this recommendation is extrapolated from studies in adults. Retrospective observational studies have shown successful rewarming as well as faster rewarming for adults who are not in cardiac arrest with core temperature less than 28 °C (82.4 °F) with ECLS compared with conventional methods. 22,24,35 A multicenter prospective observational study showed improved outcomes with use of ECMO for adults in hypothermic cardiac arrest; however, patients with hemodynamic instability without cardiac arrest who received ECMO had decreased event-free days (ICU, ventilator, and catecholamine administration) and increased hemorrhagic complications without improving survival or neurological outcomes.21
- Defibrillation before rewarming can be successful in adults and adolescents.18,20,36,37 The hypothermic heart may be less responsive to defibrillation, and the temperature at which defibrillation should first be tried in the severely hypothermic child and the number of defibrillation attempts that should be made are not well studied. If VT or VF persists, excessive defibrillation attempts may lead to myocardial injury. In a large retrospective observational study, while a shockable initial rhythm in adult patients with hypothermic OHCA was associated with better survival and neurologically favorable survival, prehospital defibrillation and increasing the number of defibrillation attempts were not.38 However, only a small number of adults with shockable rhythms were not defibrillated, so the study may have been underpowered to detect a difference in outcomes in this population. The Wilderness Medical Society guideline recommends defibrillating once initially and deferring additional defibrillation attempts until core temperature reaches 30 °C (86 °F) because these are more likely to be successful.39,40 ERC guidelines allow up to 3 defibrillation attempts before rewarming to 30 °C (86 °F).41 There are no relevant societal guidelines specific to children, and, thus, recommendations are extrapolated from adult data.
- The temperature at which epinephrine should first be administered and the optimal dosing interval in the severely hypothermic child has not been well-studied. Some adult and adolescent cases of hypothermic cardiac arrest have normal responses to epinephrine before rewarming.18 However, hypothermic hearts may be less responsive to cardiovascular drugs and slowed metabolism due to hypothermia raises concerns for epinephrine accumulation and toxicity.41,43,44 There are no relevant societal guidelines specific to children, and, thus, recommendations are extrapolated from adult data.
- In cases of prolonged CPR or dangerous environmental conditions, it may be suboptimal or impossible to perform continuous high-quality CPR. Delayed or interrupted CPR may facilitate rapid extraction or transportation and allow for optimal CPR as soon as feasible or safe. Adult cases with delayed or intermittent CPR have led to successful resuscitations, but there are no reported cases in children.45 The acceptable frequency and duration of CPR interruptions and which children can best tolerate this are unknown.
- Rapid hospital transport is important to allow for aggressive rewarming with both noninvasive and invasive techniques. Two studies utilizing the same database conflicted on the association of low-flow time (time from cardiac arrest to the establishment of ECMO) with outcomes for adults in cardiac arrest due to hypothermia, but there are no such studies in children.46,47 Adults have been resuscitated using ECLS after hypothermic cardiac arrest lasting over 6 hours, so the ERC supports transporting to an ECLS-capable center if within 6 hours.41,48,49 An observational study showed that transportation to centers with ECLS capability improved neurologically favorable outcomes and 1-month survival in adults.38 There is no data in children, and, thus, the recommendation is extrapolated from adult data.
- ETCO2 measurement by waveform capnography is utilized for confirmation of correct endotracheal tube placement and monitoring ventilation during endotracheal intubation. For children in nonhypothermic cardiac arrest, ETCO2 is often used to monitor and optimize CPR quality and an abrupt increase in CO2 can indicate ROSC. In an observational study comparing adults with hypothermic with nonhypothermic cardiac arrest assessed for ECLS, those in hypothermic cardiac arrest had significantly lower ETCO2 and temperature was positively correlated with ETCO2.27 Also, 45% of adult survivors had ETCO2 ≤10 mm Hg at hospital admission, and the value of ETCO2 did not predict survival.27 There are no studies available in children, and, thus, recommendations are extrapolated from adult data.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In unresponsive adults and children with durable LVADs and impaired perfusion, chest compressions should be performed. |
| 2b | C-EO | 2. In unresponsive adults and children with durable LVADs and impaired perfusion, it may be reasonable to start chest compressions immediately while simultaneously assessing for device-related reversible causes. |
| 2b | C-EO | 3. In unresponsive adults and children with durable LVADs and impaired perfusion, it may be reasonable to utilize an algorithmic approach to concurrently assess and respond to impaired perfusion. |
Synopsis
LVADs deliver blood from the weakened left ventricle to the systemic circulation.1 A durable LVAD comprises an inflow from the apex of the left ventricle, an implanted electrical pump, an outflow to the ascending aorta, a percutaneous driveline connection, and an external controller powered by batteries (Figure 3). The only currently implanted durable LVAD in adults and children in the United States has a centrifugal pump (eg, HeartMate 3 LVAD [Abbott]) and is fully magnetically levitated to prevent mechanical wear and decrease the risk of thrombosis. There are patients living with older durable LVADs, such as HeartMate II (Abbott) and HeartWare Ventricular Assist Device (Medtronic).
Cardiac arrest can occur in people with durable LVADs in cases of device malfunction, inadequate device flow, or low cardiac output of the unsupported right ventricle, whereas some typical causes of cardiac arrest, such as ventricular arrhythmias, may be better tolerated.2,3 Current durable LVADs have continuous rather than pulsatile flow, so most patients do not have a palpable pulse, making detection of cardiac arrest difficult. Additionally, automated noninvasive blood pressure and pulse oximetry can be unreliable. Perfusion is considered adequate if any of the following is present: (1) normal skin color and temperature; (2) normal capillary refill; (3) mean arterial pressure greater than 50 mm Hg; (4) partial pressure of end-tidal carbon dioxide greater than 20 mm Hg. (Figure 4). An unresponsive patient with an LVAD without adequate perfusion is likely in cardiac arrest.
Potential concerns related to performing chest compressions in adults and children with LVADs are device dislodgement and ineffective or retrograde blood flow through a valveless LVAD during chest compressions.
These recommendations inform the management of adults and children with durable LVADs in cardiac arrest beyond the index placement hospitalization. Management of cardiac arrest in the peri-implantation period is discussed in the recommendations detailed in the Cardiac Surgery section.
Recommendation-Specific Supportive Text
- There are no RCTs or high-quality observational studies comparing chest compressions versus not performing chest compressions in adults or children with an LVAD. The largest study, a retrospective cohort of 578 adults with LVADs with cardiac arrest, showed patients who received chest compressions had higher in-hospital mortality than those who did not, and CPR was an independent predictor of mortality.4 In another retrospective cohort study including 58 adults with LVADs who had cardiac arrest, there was no difference in survival in those receiving chest compressions versus those not receiving compressions.5 In a case series of 16 adults, 2 of 9 (22.2%) patients who received chest compressions survived to discharge compared with 3 of 7 (42.9%) who did not receive chest compressions.6 There is high risk of bias in these retrospective studies, with potentially poorer outcomes occurring in patients receiving chest compressions due to a delay in initiation in this patient population and failure of this subset of patients to respond to other ALS interventions, such as defibrillation or epinephrine. There is also high risk of misclassification of patients as having a cardiac arrest when not truly in cardiac arrest. There are many case reports of successful use of chest compressions in adults and children with an LVAD.7-14 No study reported dislodgement or other LVAD device dysfunction after chest compressions. In 1 study, 3 adults underwent autopsy to confirm the LVAD was in appropriate position, including 1 patient who underwent 2.5 hours of chest compressions.15 Despite the mechanical left ventricular support provided by an LVAD, CPR can provide temporary circulatory support in cardiac arrest resulting from device malfunction or right ventricular failure. CPR in the absence of LVAD saves lives, and poor outcomes may have resulted from hesitancy to start CPR in this patient population. The benefit of immediate initiation of CPR in unresponsive adults and children with LVADs and impaired perfusion outweighs the risk.
- Multiple case series and case reports have reported delays in initiation of chest compressions in adults with an LVAD, and delays were longer for patients with an LVAD compared with controls without an LVAD.6,9,10,12 The most common reason for clinicians not performing chest compressions in patients with an LVAD with acutely impaired perfusion was a belief that chest compressions were contraindicated in this population.5 Delays in chest compressions have been highlighted as a potential contributor to poor outcomes.5,9 While the British Societies recommend delaying chest compressions in patients with an LVAD for up to 2 minutes while attempting to restart the device, these efforts could occur in parallel to prevent delays in chest compressions per the 2024 ILCOR scoping review (Figure 4).16
- Because of the difficulty assessing perfusion and uncertainty regarding the safety of chest compressions in adults and children with an LVAD, several studies and reviews have proposed algorithms for the resuscitation of this population (Figure 4).1,6,9,14,17,18 Signs and symptoms related to poor tissue perfusion, such as altered mentation or unresponsiveness, agonal breathing or apnea, cool temperature, and delayed capillary refill, are important for initial patient evaluation. The primary consideration for a patient with an LVAD with acutely altered perfusion is whether the device is functioning, and this can be assessed by auscultating for a mechanical ventricular assist device hum over the left chest and left upper abdomen.1 Troubleshooting an LVAD includes assessment of the controller for alarms, assessment of the driveline for disconnection or fracture and reconnecting if possible, and ensuring an adequate power source connection.17 Doppler with a manual cuff or an arterial line can more reliably measure blood pressure in a patient with an LVAD, and waveform capnography can aid in assessment of perfusion. There are no studies demonstrating improved outcomes with utilization of an algorithm for managing adults or children with a durable LVAD in cardiac arrest.

Figure 4. Adult and Pediatric Durable LVAD Algorithm.
Abbreviations: ALS, advanced life support; BLS, basic life support; BP, blood pressure; ET, endotracheal; LVAD, left ventricular assist device; MAP, mean arterial pressure; PETCO2, partial pressure of end-tidal carbon dioxide; VAD, ventricular assist device.
Cardiac arrest in pregnancy is infrequent, but the rate appears to be increasing.1,2 Because of the relative rarity of the event, the exclusion of pregnant people from clinical trials, and ethical considerations, most studies addressing cardiac arrest in pregnancy are observational. Variability in the populations included in these studies further challenges the interpretation of literature. The term obstetrics encompasses pregnancy, childbirth, and postpartum states, whereas the term peripartum includes late pregnancy, intrapartum, and immediate postpartum periods. Existing recommendations center on maintaining best resuscitation practices with modifications to account for the physiologic changes of pregnancy and address underlying etiologies specific to obstetric patients.1,3,4 Reversible causes of cardiac arrest in pregnancy parallel those in the nonobstetric population in terms of the underlying pathophysiology (eg, hypovolemia, hypoxemia, thrombosis). A pregnancy-specific mnemonic (Figure 5) emphasizes the leading causes of death in obstetric patients (eg, hemorrhage, sepsis, and hypertension) while highlighting diagnoses unique to, or commonly encountered in, the peripartum period (eg, amniotic fluid embolism, anesthetic complications, cardiomyopathy, and medications such as magnesium infusions).
Reported rates of survival for the pregnant patient and newborn infant vary widely, reflecting differences in the causes of cardiac arrest, readiness of the clinical team, resources available, location, and gestational age at the time of the event.1,4,5 Optimizing outcomes for both the pregnant patient and newborn infant requires advanced planning to ensure the clinical team can provide timely and effective resuscitation.6
Guidelines for the resuscitation of newborn infants are provided in “Part 5: Neonatal Life Support.” Ethical considerations of performing resuscitation on pregnant patients are discussed in “Part 3: Ethics.”
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Establishing a team prepared to manage cardiac arrest in a pregnant person is recommended. |
| 1 | C-LD | 2. The resuscitation team should access resources, including health care professionals, their expertise, and the necessary equipment, to perform a resuscitative delivery at the diagnosis of cardiac arrest in a pregnant patient. |
| 1 | C-LD | 3. Role-specific training should be provided for health care professionals with the potential to respond to cardiac arrest in a pregnant patient. |
Synopsis
The management of cardiac arrest in a pregnant patient requires modifications to account for the physiology of pregnancy, unique causes of cardiac arrest, and the potential need for a resuscitative delivery if ROSC cannot be achieved. Essential roles of a resuscitation team include performing left lateral uterine displacement; providing high-quality, minimally interrupted CPR; managing the difficult airway; and performing a resuscitative delivery.3 If resuscitative delivery is effective in achieving ROSC, teams may be tasked with the medical and surgical management of life-threatening postpartum hemorrhage. Resuscitative delivery may lead to the birth of a viable newborn infant in need of resuscitation, which will ideally be provided by a dedicated team experienced with the resuscitation of term and preterm infants. Timely access to the resources and personnel capable of coordinating this multidisciplinary endeavor will vary based on the local environment. The complexity of this clinical scenario underscores the need for teams to prepare and train for this situation.
These recommendations inform team preparation by facilities capable of responding to and treating pregnant patients in cardiac arrest.
Recommendation-Specific Supportive Text
- Team composition will vary according to local resources but may include (1) a team leader skilled in the management of cardiac arrest and aware of pregnancy-specific modifications; (2) an anesthesiologist or other operator familiar with intubating pregnant patients and supporting massive transfusion; (3) an obstetrician or surgeon with experience performing cesarean deliveries and surgically managing hemorrhage; (4) a designated neonatal resuscitation team; and (5) nurses, pharmacists, and other professionals skilled at IV access, medication selection, medication administration, and other key resuscitation tasks.3 Health care professionals capable of performing cannulation for VA-ECMO may play a role in settings where this resource is available. An analysis of 561 cases of IHCA using the Get With The Guidelines-Resuscitation registry demonstrated an association between the location of cardiac arrest within the hospital and survival.5 A cardiac arrest occurring in the ICU or post anesthesia care unit conferred a 3 times increased adjusted odds of mortality (aOR, 3.32; 95% CI, 2.00–5.52) compared with those with a cardiac arrest occurring in the labor and delivery unit. This association could reflect residual confounding based on morbidity burden, but it may also reflect lack of readiness of the team for cardiac arrest in pregnancy.
- Despite longstanding guidelines recommending that a resuscitative delivery (historically referred to as a perimortem cesarean delivery) be initiated if ROSC is not achieved within 4 minutes of cardiac arrest, in most cases this intervention is not performed in the recommended timeframe.3,7-11 Institutions and organizations that may be required to manage cardiac arrest in a pregnant patient can prioritize developing, training, and implementing processes to perform a resuscitative delivery rapidly and safely.6 In cases of IHCA, this will include summoning an obstetrician or surgeon, if available, prepared to perform a resuscitative delivery along with requisite tools for this procedure as well as the necessary equipment for neonatal resuscitation. Optimal management of OHCA during pregnancy poses significant clinical challenges and requires performing high-quality resuscitation while rapidly transporting the pregnant patient to a center capable of safely performing the procedure. A population-based study from France evaluated 55 pregnant patients with OHCA and reported that 30-day survival was 14.5% with neonatal survival rates of 10.0% when cardiac arrest occurred in the second trimester and 23.5% in the third trimester.12 This underscores the potential for a good outcome for the patient and newborn infant when effective resuscitation is provided.
- Population-based reviews of resuscitation in pregnant patients highlight opportunities for improvement. A population-based surveillance study of cardiac arrest in pregnancy from the Netherlands demonstrated that less than a third of pregnant patients (4/14 [29%]) received maneuvers to alleviate aortocaval compression.13 A study from the United Kingdom found similar results with only 44% (n=29/66) receiving maneuvers to relieve aortocaval compression.14 Data from the Netherlands demonstrated improved rates of appropriate resuscitative delivery before and after implementation of the Managing Obstetric Emergencies and Trauma course for health care professionals in the country (0.36/year versus 1.6/year; P15 Simulation-based studies demonstrate improved knowledge of cardiac arrest in pregnancy, underscoring a need for training as a key step to improve readiness.6,16,17
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Resuscitative delivery is indicated for a pregnant patient in cardiac arrest when fundal height is at or above the umbilicus and ROSC is not achieved with standard resuscitation. |
| 1 | B-NR | 2. Preparation for resuscitative delivery for a pregnant patient in cardiac arrest should begin at the recognition of cardiac arrest, with the goal to complete delivery by 5 minutes. |
| 1 | C-LD | 3. Resuscitative delivery for a pregnant patient with IHCA should be performed at the location of the arrest, without attempts to move the pregnant patient to the operating room. |
| 1 | C-LD | 4. Manual left lateral uterine displacement should be provided in conjunction with chest compressions for a pregnant patient in cardiac arrest when the fundal height is at or above the umbilicus. |
| 1 | C-LD | 5. Airway management should be prioritized during the resuscitation of a pregnant patient in cardiac arrest. |
| 2a | B-NR | 6. It is reasonable to use extracorporeal cardiopulmonary resuscitation in pregnant or peripartum patients in cardiac arrest not responsive to standard resuscitation. |
| 2a | C-EO | 7. Placing IV or IO access in the upper extremities as opposed to the lower extremities is reasonable for a pregnant patient in cardiac arrest. |
| 2a | C-EO | 8. Administration of calcium to a pregnant patient who develops cardiac arrest while receiving IV magnesium sulfate is reasonable. |
| 3: No Benefit | C-EO | 9. Fetal monitors should be removed, and fetal monitoring should not be performed for a pregnant patient in cardiac arrest. |
Synopsis
Modifications to resuscitation in the pregnant patient with cardiac arrest are based on the anatomic and physiologic changes of pregnancy.3 The growing uterus can cause position-dependent aortocaval compression when the pregnant person is supine. Compression of the inferior vena cava leads to a decrease in preload, stroke volume, and cardiac output, which must be overcome to enable adequate blood flow.18
The increased oxygen consumption in the pregnant person underscores the importance of ensuring adequate oxygenation and ventilation as a part of resuscitation. Pregnancy is characterized as a compensated respiratory alkalosis driven by an increased tidal volume with metabolic compensation. The increased tidal volume with the enlarging uterus results in a lower functional residual capacity. The decrease in functional residual capacity along with increased oxygen demand leads to a greater risk of hypoxemia during advanced airway placement.3
If interventions to enhance oxygen delivery and reverse underlying causes do not achieve ROSC, attention is then focused on increasing forward blood flow and decreasing oxygen demand by delivering the fetus. The primary purpose of delivery is for resuscitation of the pregnant patient by relieving aortocaval compression and by returning blood sequestered in the uterus to the systemic circulation.19 Improving neonatal prognosis by limiting intrauterine hypoxemia and its consequences is the secondary benefit of this intervention, noting that fetal survival is dependent on either maternal survival or resuscitative delivery.8
These recommendations inform the management of pregnant patients in cardiac arrest.
Recommendation-Specific Supportive Text
- The term resuscitative delivery has replaced the historical term perimortem cesarean delivery to reflect its central role in the resuscitation of the pregnant patient and to support attempts to avoid unnecessary surgery when feasible.10 Resuscitative delivery most commonly occurs via laparotomy and hysterotomy (eg, cesarean delivery), but delivery can be achieved via assisted vaginal delivery if the cervix is fully dilated at the time of cardiac arrest. Associating the performance of resuscitative delivery and improved outcomes is challenging because selection bias favors better outcomes for those who achieve ROSC early enough in resuscitation that the procedure is no longer needed. Case series, a meta-analysis of case reports, and a prospective observational study all demonstrate an increased likelihood of survival of the pregnant patient and the newborn infant with shorter time intervals between cardiac arrest and resuscitative delivery.8,14,20
- Resuscitative delivery initiated at 4 minutes and completed by 5 minutes from the onset of cardiac arrest was first proposed in 1986 and has remained the recommended time interval for this procedure based on practicality and available evidence.21 From a pragmatic standpoint, a time interval of 4 minutes allows for 2 rounds of CPR including administration of epinephrine, defibrillation in the setting of a shockable rhythm, and attempts to address reversible causes. A population-based study evaluating cardiac arrest in obstetric hospitals in the United Kingdom demonstrated a shorter time interval between cardiac arrest and resuscitative delivery in surviving pregnant patients compared with nonsurvivors (3 versus 12 minutes; P=0.001).14 In a systematic review of 94 cases of cardiac arrest, resuscitative delivery within 10 minutes of cardiac arrest was associated with 5 times increased odds of survival of the pregnant patient.8 In the subgroup analysis of 57 people undergoing resuscitative delivery, time from arrest to delivery was shorter in neonatal survivors compared with nonsurvivors (14 versus 22 minutes; P=0.02).8 This was supported by a review of published cases, which found a consistent association between injury-free survival for the pregnant patient and newborn infant and early resuscitative delivery. No delivery time inflection point or cut-off time was found.7
- Moving the pregnant patient from the site of cardiac arrest for resuscitative delivery was associated with lower rates of ROSC in a small observational study, though results were potentially confounded by the inclusion of OHCA cases, which have overall poorer prognosis.20 A simulation-based study randomized multidisciplinary teams to perform resuscitative delivery at the location of the arrest or movement to the operating room for resuscitative delivery. The teams performing the delivery at the location of the arrest achieved delivery 3.5 minutes faster with fewer disruptions in chest compressions compared with those moving to the operating room for delivery.22 In a simulation study evaluating the impact of transport on the quality of cardiopulmonary resuscitation, teams randomized to transporting the manikin to the operating room showed lower rates of correct chest compressions with higher rates of interruption compared with the group randomized to stay in the location of simulated cardiac arrest.23
- Manual left lateral uterine displacement (Figure 6) alleviates aortocaval compression while allowing the patient to remain in a supine position during the resuscitation. A prospective cohort study used quantitative cardiac magnetic resonance imaging to demonstrate that pregnant people have decreased cardiac output in the supine position compared with the left lateral position at 20 weeks and 32 weeks of pregnancy.18 Left ventricular end diastolic volume, left atrial volume, and stroke volume were higher in the left lateral compared with supine position, supporting the ability of position to increase preload and cardiac output. A meta-analysis including 8 simulation-based crossover trials demonstrated superior chest compression technique when the manikin was placed in a supine as opposed to a left lateral tilt position.24 One of the included studies used a porcine model to demonstrate improved coronary perfusion pressure when chest compressions were performed when supine with left lateral uterine displacement versus supine positioning alone.25
- The physiologic changes of pregnancy render pregnant people less tolerant of apneic oxygenation and more vulnerable to hypoxemia, hypercarbia, and subsequent acidosis than other people in cardiac arrest. Providing adequate oxygenation and ventilation optimizes the resuscitation of the pregnant patient while maintaining favorable gradients of oxygen and carbon dioxide across the uteroplacental interface, which is critical to neonatal outcomes.3 Anatomic and hormonal changes increase the risk of aspiration in pregnancy, making endotracheal intubation ideal for airway management, but the likelihood of airway edema contributes to higher rates of difficult or failed intubation in pregnant patients.26,27 The “difficult” airway is navigated with a smaller endotracheal tube by a team member with expertise in laryngoscopy and consideration of a supraglottic airway after 2 failed intubation attempts.3,28
- Theoretical concerns about bleeding and thrombosis in obstetric patients have delayed the use of ECLS in peripartum patients, but emerging data support its use. A systematic review of 358 obstetric patients managed with ECLS reported a 30-day survival rate of 75%.29 Fifty-seven of these patients were cannulated for the indication of cardiac arrest during pregnancy or in the first 24 hours after delivery with an 87% 30-day survival rate.29 Only 13% of patients in this study experienced severe bleeding necessitating laparotomy or hysterectomy, with a 3% rate of venous thromboembolism. A 2020 analysis of 277 obstetric patients in the ELSO Registry found a 55% rate of survival among the 42 patients receiving ECLS for the indication of cardiac arrest.30 This 55% survival rate among obstetric patients cannulated for an indication of cardiac arrest compares favorably with the 29% rate of survival for patients receiving ECPR in the ELSO Registry over a similar time period.31 ELSO guidelines suggest summoning available resources, including health care professionals, their expertise, and the necessary equipment, for evaluation for possible ECPR after 10 to 15 minutes of standard resuscitation without ROSC.32
- Although not directly studied, aortocaval compression likely impedes delivery of resuscitation drugs administered in the lower extremities to the central circulation. A radionucleotide study in nonpregnant canines found that drugs instilled into the superior vena cava reached the heart faster than drugs instilled into the inferior vena cava.32 However, studies from OHCA (excluding pregnant people) have not consistently demonstrated favorable neurologic outcomes when IO devices were placed in the upper extremity compared to the lower extremity.33-35
- Magnesium sulfate is a commonly utilized medication in obstetrics, administered as a bolus dose followed by a continuous infusion for management of preeclampsia and for other specific patients at risk of preterm delivery. Life-threatening and fatal hypermagnesemia due to medication error has been reported in obstetric and nonobstetric patients.36-38 A meta-analysis examining adverse effects from magnesium sulfate administration stratified by indication and regimen found an increased risk of respiratory depression in patients with preeclampsia receiving magnesium.36 A study of patients on magnesium infusions for obstetric indications demonstrated that increasing concentrations of serum magnesium were associated with lower ionized calcium concentration and increased urinary calcium excretion.39 Calcium administration may improve hemodynamics in the setting of magnesium toxicity, informing its use in cardiac arrest.40 Most patients in case reports received calcium along with multiple concurrent interventions, limiting the usefulness of these reports.
- The physiologic dependence of the fetus on the circulation of the pregnant patient coupled with the high survival rate of newborn infants in the setting of ROSC prior to resuscitative delivery underscores the importance of prioritizing resuscitation of the pregnant patient.8 Fetal monitoring will not change management and may worsen outcomes by distracting the team and limiting timely defibrillation and resuscitative delivery when indicated.3 Removal of external and internal fetal monitors at the diagnosis of cardiac arrest avoids these distractions and minimizes risk of rescuer injury in the setting of defibrillation.
The 2025 treatment algorithm for the management of cardiac arrest in pregnancy is shown in Figure 5.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. A massive transfusion protocol with a balanced transfusion strategy should be used for peripartum patients with life-threatening suspected amniotic fluid embolism. |
| 1 | C-LD | 2. Tranexamic acid should be administered to peripartum patients with life-threatening suspected amniotic fluid embolism. |
| 2a | C-LD | 3. It is reasonable to use VA-ECMO for peripartum patients with life-threatening suspected amniotic fluid embolism. |
| 2a | C-EO | 4. Use of inhaled pulmonary vasodilators is reasonable for peripartum patients with life-threatening suspected amniotic fluid embolism. |
| 3: No Benefit | C-EO | 5. Use of atropine in the absence of bradycardia is not recommended for peripartum patients with life-threatening suspected amniotic fluid embolism. |
Synopsis
Amniotic fluid embolism (AFE) is a clinical diagnosis characterized by sudden hemodynamic and respiratory compromise during labor or shortly after delivery that can lead to cardiac arrest.41 The presence of disseminated intravascular coagulation and resultant hemorrhage is a hallmark feature of the disease.42,43 A key feature in the pathophysiology of AFE involves exposure to fetal antigens with proteins, such as tissue factor and endothelin.41 A complete understanding of the molecular mechanism triggering this hyperimmune response is still a topic of investigation, but the historic perspective of the pathophysiology of AFE characterized by fetal cells obstructing the pulmonary vasculature is incorrect.44,45
Tremendous overlap in the clinical features of AFE and other common causes of cardiac arrest make a high index of suspicion, timely diagnosis, and interventions addressing the underlying pathophysiology of critical importance.46 Echocardiography evaluating for increased pulmonary vascular resistance and right ventricular failure along with viscoelastic testing assessing for hyperfibrinolysis have been proposed to aid in timely diagnosis.47-50 National guidelines for the diagnosis vary without a reference standard clinical or pathologic diagnosis.51
These recommendations inform the management of pregnant patients with life-threatening AFE, including cardiac arrest.
Refer to Figure 5 for pregnancy modifications to standard ALS.
Recommendation-Specific Supportive Text
- Balanced transfusion characterized by the transfusion of red blood cells, fresh frozen plasma, and platelets equivalent to the constitution of whole blood is a cornerstone in the management of active bleeding and hemorrhagic shock in obstetric and nonobstetric patients.52,53 A massive transfusion protocol is an institution-specific response to expedite access to support a large-volume transfusion of whole blood components in the setting of life-threatening hemorrhage.53 The pathophysiology of hyperfibrinolysis alongside population-level data from multiple countries support the potential need for this intervention in the setting of suspected AFE.53,54 A population-based study evaluating cases of suspected AFE in France reported 10 units of red blood cells as the median transfusion volume (range, 0–22 units) while also noting 16 of 36 people received suboptimal hemorrhage management.55 The importance of a balanced transfusion strategy that replaces coagulation factors along with red blood cells is underscored in a multinational nested case-control study evaluating the association between hemorrhage management and death in the setting of suspected AFE.51 An analysis of 216 subjects identified by the United Kingdom Obstetric Surveillance System demonstrated a reduced risk of death in the setting of transfusion of concentrated fibrinogen (aOR, 0.44; 95% CI, 0.21–0.92) and platelets (aOR, 0.23; 95% CI, 0.10–0.52), noting the importance of coagulation factors in addition to red blood cells.
- Tranexamic acid (TXA) works through competitive binding to limit the activation of plasmin—the enzyme that prevents excess clot formation in physiologic states by cleaving fibrinogen to fibrin and its split products. The hyperfibrinolysis of disseminated intravascular coagulation destabilizes existing blood clots while generating fibrin split products that cause microvascular injury. Therefore, the mechanism of action of TXA is to prevent clot degradation and downstream microangiopathy—not to promote clot formation. Two RCTs of more than 20 000 adult patients each demonstrated a mortality benefit with TXA administration in the setting of hemorrhage. In the CRASH-2 trial, receipt of TXA reduced the risk of death in the setting of trauma without an increase in venous thromboembolism.56 The WOMAN trial showed a mortality benefit in obstetric patients with postpartum hemorrhage, but not specifically AFE, randomized to receive TXA.57 International guidelines from the World Health Organization support the use of TXA in the setting of postpartum hemorrhage, with recommendations from the Society for Maternal-Fetal Medicine underscoring its unique role in the management of suspected AFE.58,59
- ECLS with VA-ECMO has been used as a bridge to recovery in case reports and case series of peripartum women with suspected AFE refractory to medical management.60,61 A retrospective case series from 2 high-volume ECMO centers in France evaluated outcomes for the 10 peripartum patients with suspected AFE placed on VA-ECMO over a 13-year period.60 Seven of the 10 patients suffered from cardiac arrest, 2 of whom were cannulated during ECPR. Seven of the patients were alive at hospital discharge, representing 70% survival in this case series. An ELSO Registry study reported a 62% rate of survival among the 34 patients placed on VA-ECMO in the setting of AFE over a 5-year period.62 Reported outcomes vary in the setting of AFE, with estimated survival rates predating more widespread use of ECMO ranging between 11% and 44%.63 The comparatively high rate of survival in patients with suspected AFE receiving ECLS suggests a role for this therapy as a bridge to recovery in this rare disease.
- Inhaled pulmonary vasodilators selectively dilate pulmonary capillaries receiving alveolar gas exchange without causing systemic vasodilation, and case reports have described their use for the management of pulmonary hypertension during labor and delivery.64-66 A case report of the use of inhaled nitric oxide demonstrated rapid reversal of hemodynamic compromise in the setting of AFE.67 These limited data alongside biologic plausibility supporting the use of inhaled nitric oxide in the unique pathophysiology of AFE suggests a potential role for this therapy in this life-threatening disease.53
- Case reports have highlighted the use of atropine in conjunction with ondansetron and ketorolac for targeted therapy for peripartum patients with AFE.68,69 These case reports include patients receiving other interventions and hemodynamic support making the contribution of this novel regimen to the reported outcome uncertain. Serotonin and thromboxane antagonism with ondansetron and ketorolac, respectively, manifest limited hemodynamic consequence.68 Atropine has been included in this pathway regardless of the presenting rhythm, presumably because of its role as a pulmonary vasodilator or its chronotropic effect.70 Atropine may cause nonselective pulmonary vasodilation, potentially worsening ventilation and perfusion mismatch. The increased heart rate limits diastolic filling time, which can impact the coronary and systemic circulations, particularly in settings of right ventricular pressure overload. The lack of benefit alongside the availability of other vasoactive agents capable of providing chronotropic and inotropic support inform this recommendation.
PE is the occlusion of 1 or more pulmonary arteries and is a potentially reversible cause of hemodynamic instability or cardiac arrest. Partial or complete occlusion of pulmonary arteries results in ventilation and perfusion mismatch and increases in pulmonary artery pressures, causing impaired right ventricular function and, ultimately, cardiovascular collapse.1,2 In 1 large review of hospitalized patients diagnosed with PE, mortality of hemodynamically unstable patients was 76.6% and of hemodynamically stable patients was 10.8%, highlighting the severity of this disease process.3 Mortality in OHCA secondary to PE is reported as high as 91%.4 Most patients in cardiac arrest secondary to PE exhibit PEA (26%–75%) or asystole (19%–73%).4-6 Treatment of patients in cardiac arrest from PE include systemic and catheter-guided fibrinolysis, surgical and mechanical embolectomy, and ECMO. Each augments resuscitation by restoring pulmonary and systemic circulation.
Τhe diagnosis of PE in children is often delayed because of diagnostic challenges and differences in symptomatology compared with adults.7 Considering children are able to compensate for shock states with increased systemic vascular resistance, hypotension is a late sign of shock in children.7 There are limited data available and no RCTs to inform the care of children.
These recommendations are modifications to standard ACLS for adults and children in cardiac arrest from confirmed or suspected PE. The treatment of patients with life-threatening PE not in cardiac arrest are outside of the scope of these recommendations, although several studies cited in the recommendations contain overlapping populations of hemodynamically unstable patients with PE and patients with PE in cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. For adults with confirmed PE as the precipitant of cardiac arrest, systemic fibrinolysis, surgical embolectomy, and percutaneous mechanical embolectomy are reasonable treatment options. |
| 2a | B-NR | 2. It is reasonable to use ECLS in adults with cardiac arrest from confirmed or suspected PE. |
| 2b | B-NR | 3. For adults in cardiac arrest from suspected PE, systemic fibrinolysis may be considered. |
| 2b | C-EO | 4. The optimal duration of CPR after administration of systemic fibrinolytic to adults in cardiac arrest from confirmed or suspected PE is unclear. |
Recommendation-Specific Supportive Text
- The treatment of adults in cardiac arrest from a confirmed PE may differ between institutions depending upon the available resources. In 1 retrospective multicenter study of 104 adult patients, including 70 patients in cardiac arrest who received systemic fibrinolytics, patients with confirmed PE had significantly greater survival to hospital discharge compared with those with a presumed PE (26% versus 4%; P=0.01).8 A review of 153 adults who experienced cardiac arrest from PE who underwent surgical embolectomy showed 56% survival.9 One retrospective case series of hemodynamically unstable adults with confirmed PE, including patients who required CPR, showed lower in-hospital mortality for those who underwent percutaneous mechanical pulmonary embolectomy compared with no embolectomy (64.4% versus 76.8%; P less than 0.001).3 The benefit of systemic fibrinolysis is weighed against the risk of bleeding complications. One study of adults with OHCA from PE observed the same risk of death from hemorrhage in patients who were treated with systemic fibrinolytics compared with those who were not (6% versus 5%; P=0.73).4 Another study observed 37.9% of adults developed bleeding complications, including intracranial hemorrhage in 9.1% of adults.10
- ECLS, including VA-ECMO, for adults in cardiac arrest has become increasingly available.11 In 1 retrospective before-and-after implementation study of ECPR in 39 adults who suffered cardiac arrest due to suspected or confirmed PE, 19 underwent VA-ECMO cannulation during CPR.12 Adults who received VA-ECMO during CPR had higher ICU survival (26% versus 5%; P less than 0.01) and higher survival with favorable neurologic outcomes (21% versus 0%; P less than 0.05) than those patients receiving conventional CPR.12 In a systematic review and meta-analysis of 77 studies including 301 adults who suffered cardiac arrest from PE, 65% of patients placed on VA-ECMO during CPR survived to hospital discharge.13 Two separate retrospective reviews of adults treated with venovenous ECMO or VA-ECMO for hemodynamic instability or cardiac arrest from confirmed PE showed no difference in survival rate in adults who received unfractionated heparin alone compared with those who received systemic fibrinolytics or surgical or interventional thrombectomy.14,15 One meta-analysis showed no increased risk of mortality for adults who received systemic fibrinolysis prior to VA-ECMO or venovenous ECMO cannulation.13
- Investigating the efficacy of systemic fibrinolytics to treat suspected PE is challenging due to variations in patient enrollment, which often lead to the inclusion of many patients with cardiac arrest from causes unrelated to PE. The definition of suspected PE in reviewed studies is not well-defined, and research is conflicting. Two RCTs found that administration of systemic fibrinolytics did not improve survival in adults and children (greater than 16 years of age) with undifferentiated cardiac arrest.16,17 In 1 retrospective registry study of 58 adult OHCA patients treated with systemic fibrinolysis for suspected PE, systemic fibrinolytics provided no survival benefit at 24 hours versus control (66% versus 63%; P=0.76); however, a 30-day mortality benefit was identified after the population was appropriately weighted (P=0.005; adjusted log-rank test).4 Two additional retrospective investigations of adults with confirmed or suspected PE as a cause of cardiac arrest showed no increased rates of ROSC or 30-day survival with systemic fibrinolytics.10,18
- There are no trials comparing different durations of continued resuscitation following systemic fibrinolysis administration to adults in cardiac arrest from any cause, including confirmed or suspected PE. The 2019 European Society of Cardiology guidelines for the diagnosis and management of acute PE recommend continuing CPR for at least 60 to 90 minutes after administration of a systemic fibrinolytic based upon case reports and case series of adults who survived after 90 to 100 minutes of CPR.2,19,20 There is significant variation in the duration of CPR after fibrinolysis between studies. One randomized trial investigating the use of systemic fibrinolysis to treat adults in PEA arrest continued CPR for at least 15 minutes after systemic fibrinolytic administration.16 A second randomized trial continued CPR for at least 30 minutes after systemic fibrinolytic administration to adults in cardiac arrest.17
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. For children with confirmed PE as the precipitant of cardiac arrest, systemic fibrinolysis, surgical embolectomy, and percutaneous mechanical embolectomy are reasonable treatment options. |
| 2a | C-LD | 2. It is reasonable to use ECLS in children with cardiac arrest from confirmed or suspected PE. |
| 2b | C-EO | 3. For children in cardiac arrest from suspected PE, systemic fibrinolysis may be considered. |
| 2b | C-EO | 4. The optimal duration of CPR after administration of systemic fibrinolytic to children in cardiac arrest from confirmed or suspected PE is unclear. |
Recommendation-Specific Supportive Text
- The recommendation for the treatment of children in cardiac arrest from confirmed PE is based on pediatric case series and data extrapolated from adults. One retrospective review of 33 children (less than 19 years of age) with confirmed PE included 3 children who underwent surgical embolectomy, 3 who received catheter-based therapies, and 1 who received systemic thrombolysis.7 Of these children, 4 experienced cardiac arrest, 2 survived but the specific therapies they received were not described.7 Overall, 3 of the children died of PE-related causes.7 Another retrospective review of 170 children (≤18 years of age) with confirmed PE included 25 (15%) patients with shock or cardiac arrest.21 Twenty-two of these children received systemic fibrinolysis, catheter-directed therapies, or surgical thrombectomy.21 Eleven (6%) children in this case series died of PE-related causes.21 In 1 retrospective multicenter study of 104 adults, including 70 patients in cardiac arrest who received systemic fibrinolytics, patients with confirmed PE had significantly greater survival to hospital discharge compared with those with a presumed PE (26% versus 4%; P=0.01).8 The benefit of systemic fibrinolysis is weighed against the risk of bleeding complications, which is conflicting in adult studies, ranging from 6% to 37.9% of cardiac arrest patients with suspected PE.4
- The treatment of children who experience cardiac arrest from PE with ECLS, including VA-ECMO, is limited by resources available to individual institutions and individual patient factors. The recommendation for the treatment of children in cardiac arrest with ECLS is based on a pediatric registry study and data extrapolated from adults. One review of the ELSO Registry included 54 children ≤18 years of age treated with ECMO who received a primary diagnosis code of PE.22 The number of children who experienced cardiac arrest prior to treatment with ECMO was not described, however, 13 of the included children were treated with ECPR.22 Thirty-three (61%) children in this cohort survived to hospital discharge.22 In 1 retrospective before- and after-ECPR implementation study of 39 adults who suffered cardiac arrest due to suspected or confirmed PE, 19 underwent VA-ECMO cannulation during CPR.12 Adults who received VA-ECMO during CPR had higher ICU survival (26% versus 5%; P less than 0.001) and higher survival with favorable neurologic outcomes (21% versus 0%; P less than 0.05) than those patients receiving conventional CPR.12 In a systematic review and meta-analysis of 77 studies including 301 adults who suffered cardiac arrest from PE 65% of patients placed on VA-ECMO during CPR survived to hospital discharge.13
- Investigating the efficacy of systemic fibrinolytics to treat suspected PE is challenging due to variations in patient enrollment, which often lead to the inclusion of many patients with cardiac arrest from causes unrelated to PE. The definition of suspected PE in reviewed studies is not well-defined, and research is conflicting. Two RCTs found that administration of systemic fibrinolytics did not improve survival in adults and children (greater than 16 years of age) with undifferentiated cardiac arrest.16,17 One retrospective registry study of 58 adult OHCA patients treated with systemic fibrinolysis for suspected PE found that systemic fibrinolytics provided no survival benefit at 24 hours versus control (66% versus 63%; P=0.76); a 30-day mortality benefit was identified after the population was appropriately weighted (P=0.005; adjusted log-rank test).4 Two additional retrospective investigations of adults with confirmed or suspected PE as a cause of cardiac arrest showed no increased rates of ROSC or 30-day survival with systemic fibrinolytics.10,18 Data for systemic fibrinolytics in children with cardiac arrest from suspected PE is lacking, and the recommendation is extrapolated from adult data.
- There are no trials available to inform the optimal duration of continued resuscitation following systemic fibrinolysis administration to adults or children in cardiac arrest from any cause, including PE. The 2019 European Society of Cardiology guidelines for the diagnosis and management of acute PE recommends continuing CPR for at least 60 to 90 minutes after administration of a systemic fibrinolytic based on case reports and case series of adults who survived after 90 to 100 minutes of CPR.2,19,20 There is significant variation in time to continued CPR after fibrinolysis between studies. One randomized trial investigating the use of systemic fibrinolysis to treat adults in PEA arrest continued CPR for at least 15 minutes after systemic fibrinolytic administration.16 A second randomized trial continued CPR for at least 30 minutes after systemic fibrinolytic administration to adults in cardiac arrest.17
Over 105 000 people in the United States died from drug overdose in 2023.1 More than 92% of these deaths were unintentional. Over time, the population of people with fatal drug overdose is aging; overdose death rates are increasing in people aged ≥35 years and decreasing in younger people. Although most deaths are due to synthetic and semisynthetic opioids, such as fentanyl, many different poisons are responsible for these deaths.
Poisoning in this guideline refers to the cellular injury or death that results from the exposure to an exogenous substance.2,3 Management of patients with critical poisoning, defined as those in cardiac arrest, refractory shock, or other conditions posing an imminent threat of cardiac arrest, may require special techniques and antidotes in addition to standard resuscitation care to achieve survival and recovery. For example, patients may develop hypotension from β-adrenergic receptor antagonist (β-blocker) or calcium channel antagonist (CCB) poisoning that does not respond to atropine, standard vasopressors, or cardiac pacing but is amenable to targeted therapies, such as high-dose insulin. Specific antidotes are available for many life-threatening poisonings but are only safe and effective if given in the proper dose and with careful patient selection (Table 4). The ideal dose is rarely known, and in many cases, equally well-supported alternative dosing strategies exist. Many poisoned patients are often ideal candidates for ECLS techniques, such as VA-ECMO, because temporary circulatory support is a bridge to survival until the poison can be eliminated, either by natural or artificial means.
Research involving poisoned patients is ethically and logistically challenging. As a result, much data about human poisoning are descriptive and observational. Variations and uncertainty in exposure, history, co-ingestions, and comorbidities make comparisons based on clinical observations challenging. High-quality animal studies broaden understanding. Applying clinical research across species is challenging, and current evidence-based medicine structures discount the contribution of animal studies to the level or certainty of evidence about an intervention.
This section of the guidelines is designed primarily for health care professionals, but opioid overdose—by far the most common cause of poisoning death—can be treated by adults and adolescents in the community with brief training.
In the United States, Canada, and much of the rest of the world, regional poison centers can provide expert treatment guidance for the management of specific poisoning cases. Each of the 55 poison centers operating in the United States and 5 in Canada is supported by board-certified medical and clinical toxicologists with specialized training in poisoning resuscitation. Poison centers provide clinical advice to clinicians and the general public about how to manage poison emergencies. A single number is used to reach the regional poison center in the United States (1-800-222-1222) and in Canada (1-844-764-7669 [1-844-POISON-X]).
We acknowledge the contributions of the authors of the 2023 American Heart Association Focused Update on the Management of Patients With Cardiac Arrest or Life-Threatening Toxicity Due to Poisoning.3
Open table in a new window.
Benzodiazepines are commonly used sedative-hypnotics for treatment of anxiety, insomnia, seizures, and withdrawal syndromes and as a component of general anesthesia and procedural sedation. Benzodiazepines are implicated in a large number of poisoning-related deaths, usually in combination with other central nervous system (CNS) depressants, such as opioids and alcohol.1,2 Benzodiazepine overdose causes CNS depression through agonist effects at the gamma-aminobutyric acid-A receptor with resultant respiratory compromise through loss of protective airway reflexes. The subsequent hypoxemia and hypercarbia cause tissue cellular injury or death.
Flumazenil, a competitive antagonist at the benzodiazepine binding site on the gamma-aminobutyric acid-A receptor, reverses CNS and respiratory depression, potentially preventing the need for intubation and mechanical ventilation.3,4 However, flumazenil administration may precipitate refractory benzodiazepine withdrawal in patients with benzodiazepine tolerance, including seizures and arrhythmias, likely by removing benzodiazepine-mediated suppression of sympathetic tone.5
Overdose with multiple drugs is common. Flumazenil may not fully reverse respiratory depression, particularly in mixed overdoses, and it may increase the risk of seizures and arrhythmias in these situations.
These recommendations inform the management of adults and children with life-threatening benzodiazepine poisoning, including cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. If combined opioid and benzodiazepine poisoning is suspected, it is reasonable to administer naloxone first (before other antidotes) in adults with respiratory depression or respiratory arrest. |
| 2a | C-LD | 2. Flumazenil can be effective in select adults with respiratory depression or respiratory arrest caused by pure benzodiazepine poisoning who do not have contraindications to flumazenil. |
| 3: No Benefit | C-EO | 3. Flumazenil has no role in cardiac arrest related to benzodiazepine poisoning in adults. |
| 3: Harm | B-R | 4. Flumazenil administration is associated with harm in adults who are at increased risk for seizures or arrhythmias. |
Adults with Benzodiazepine Poisoning
Recommendation-Specific Supportive Text
- While isolated benzodiazepine poisoning rarely causes life-threatening hypoventilation or hemodynamic instability in adults, overdoses usually occur with concomitant opioid, ethanol, or other CNS depressant poisoning.1,2 Flumazenil (Table 4) attenuates benzodiazepine-associated respiratory depression in healthy patients receiving a combination of benzodiazepines and opioids, but this only produces a partial restoration of respiratory function. Naloxone reverses opioid poisoning, which is more common and causes more significant respiratory depression than benzodiazepine poisoning.4 This is particularly important given the presence of opioid-adulterated illicit drugs2,6 (refer to the Toxicology: Opioid Overdose section).
- Flumazenil (Table 4) is safe in some low-risk presentations in adults (eg, iatrogenic overdoses during procedural sedation) and when high-risk conditions (eg, chronic benzodiazepine dependence and congestion of other dangerous substances) can be reliably excluded. One healthy adult volunteer randomized crossover study of alfentanil plus midazolam-induced respiratory depression showed that 1 mg of flumazenil significantly improved consciousness and respiratory depression over placebo.4
- Flumazenil does not directly affect cardiac rhythm or restore spontaneous circulation in adults.7
- In a meta-analysis of randomized clinical trials of adults with confirmed or suspected benzodiazepine overdose, higher rates of serious adverse effects, including seizures and arrhythmias, were observed in patients treated with flumazenil compared with standard care.5 There are few reports of flumazenil-provoked seizures in adults with preexisting seizure disorder in the absence of other risk factors.8 While the most common cardiac adverse event associated with flumazenil was supraventricular tachycardia, ventricular arrhythmias and asystole have also been reported in adults.5,9-13 The risks of flumazenil likely exceed the benefit in patients with undifferentiated coma for whom medical history and co-intoxications are unknown.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. Administration of flumazenil can be effective in select children with respiratory depression or respiratory arrest caused by pure benzodiazepine poisoning who do not have contraindications to flumazenil. |
| 2a | C-EO | 2. If combined opioid and benzodiazepine poisoning is suspected, it is reasonable to administer naloxone first (before other antidotes) in children with respiratory depression or respiratory arrest. |
| 3: No Benefit | C-EO | 3. Flumazenil has no role in cardiac arrest related to benzodiazepine poisoning in children. |
| 3: Harm | C-LD | 4. Flumazenil administration is associated with harm in children who are at increased risk for seizures or arrhythmias. |
Children with Benzodiazepine Poisoning
Recommendation-Specific Supportive Text
- Flumazenil (Table 4) has been used safely in children when there is low risk of precipitating seizures or arrhythmias. Two RCTs of healthy children who received benzodiazepines as part of a procedural anesthetic demonstrated safety with no differences in cardiorespiratory status and no seizures reported in those who received flumazenil compared with those who did not.14,15 Awakening was faster in children who received flumazenil after general anesthesia, but there was no difference with flumazenil use after conscious sedation.14,15 A prospective nonrandomized study of children receiving midazolam for conscious sedation showed 96% had a complete or partial response to flumazenil with no significant adverse events, but children with risk factors for seizures were excluded.16 In a retrospective study of 83 children with clinical poisoning who received flumazenil after alleged benzodiazepine and nonbenzodiazepine poisoning, no flumazenil-related seizures occurred, and effectiveness was not reported.17
- There are no specific studies investigating the effects of flumazenil on respiratory depression for combination benzodiazepine and opioid overdose in children, thus, the pediatric recommendation is extrapolated from the adult data.
- Flumazenil does not directly affect cardiac rhythm or restore spontaneous circulation in children.7
- There are reports of seizure recurrence after flumazenil use to reverse benzodiazepine-induced respiratory depression after prolonged seizures in children.18,19 In a meta-analysis of randomized clinical trials in mostly adults with presumed benzodiazepine overdose, higher rates of serious adverse effects, including seizures and arrhythmias, occurred with flumazenil compared with standard care alone.5 Harms were uncommon, and in most cases, readily managed. The risks of flumazenil likely exceed the benefit in children with undifferentiated coma for whom past medical history, substance use history, and the potential poison(s) involved are unknown.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. High-dose insulin or euglycemia therapy should be administered for hypotension refractory to vasopressor therapy in adults and children with life-threatening β-blocker poisoning. |
| 1 | C-EO | 2. Vasopressors should be administered for the treatment of hypotension in adults and children with life-threatening β-blocker poisoning. |
| 2a | C-LD | 3. It is reasonable to administer a bolus of glucagon followed by continuous infusion for treatment of symptomatic bradycardia or hypotension in adults and children with life-threatening β-blocker poisoning. |
| 2a | C-LD | 4. It is reasonable to use ECLS in adults with β-blocker poisoning in cardiogenic shock refractory to pharmacological interventions. |
| 2a | C-EO | 5. It is reasonable to use ECLS in children with β-blocker poisoning in cardiogenic shock refractory to pharmacological interventions. |
| 2b | C-LD | 6. It may be reasonable to use hemodialysis for life-threatening poisoning from atenolol, nadolol, or sotalol in adults with life-threatening β-blocker poisoning. |
| 2b | C-EO | 7. It may be reasonable to use hemodialysis for life-threatening poisoning from atenolol, nadolol, or sotalol in children with life-threatening β-blocker poisoning. |
| 2b | C-EO | 8. The usefulness of IV lipid emulsion (ILE) for adults and children with refractory shock due to β-blocker poisoning is uncertain. |
| 2b | C-EO | 9. It may be reasonable to administer calcium in adults and children with life-threatening β-blocker poisoning. |
Synopsis
Direct antagonism of the β-adrenergic receptors in patients with β-blocker poisoning results in hypotension from decreased cardiac contractility, bradycardia, and AV nodal blockade.1 β-blocker poisoning has also been associated with hypoglycemia.2-6 A limited number of β-blockers cause arrhythmias from blockade of cardiac sodium (eg, propranolol) or potassium (eg, sotalol) channels and vasodilation from alpha-1 adrenergic antagonism (eg, labetalol, carvedilol).7,8 Fortunately, while unintentional exposure to β-blockers in children is common, severe toxicity including bronchospasm, cardiogenic shock, hypoglycemia, seizure, and symptomatic bradycardia were not reported in 1 review of 208 children less than 6 years of age.5 No studies have evaluated the use of therapies specific to cardiac arrest due to β-blocker poisoning. Therefore, recommendations are derived from studies in poisoned patients with severe β-blocker–induced shock.
These recommendations inform the management of adults and children with life-threatening β-blocker poisoning, including cardiac arrest.
Recommendation-Specific Supportive Text
1. High-dose insulin (Table 4) improves inotropy in case series of adults and children in cardiogenic shock from β-blocker poisoning.9,10 In some case reports of adults and children, high-dose insulin therapy appears to be vasopressor sparing. Recurrence of vasopressor-resistant hypotension after insulin therapy was also reduced or stopped, although this is not reported consistently.8,9,11,12 Protocolized care reduces the risk of hypoglycemia.13,14 Hypokalemia and volume overload are additional concerns.10,13
2. Successful use of inotropes and vasopressors is described in a systematic review of descriptive studies including adults and children as well as animal studies.8 Because they are readily available and act quickly, vasopressors, such as norepinephrine and epinephrine, are almost always the initial therapy for β-blocker–induced hypotension. Other noradrenergic vasopressors, such as vasopressin, angiotensin II, amrinone, milrinone, methylene blue, and hydroxocobalamin, are not supported by sufficient evidence to support a recommendation.
3. Intravenous glucagon (Table 4) increased contractility and improved hemodynamics in case reports and case series of adults and children with β-blocker poisoning and in 1 adult trial involving a nontoxic dose of esmolol.8,11,15-17 However, 1 retrospective observational study of adults and children did not support these effects.18 The doses used to treat hemodynamic instability from β-blocker poisoning are larger than those used to treat hypoglycemia. Nausea and vomiting are common, and in 1 animal model, rapid tachyphylaxis occurred with continuous infusion.8,19
4. and 5. Adult and pediatric case reports, adult case series, and adult observational studies demonstrated VA-ECMO may be lifesaving for adults and children with persistent cardiogenic shock refractory to maximal supportive care.20-23 Limited pediatric data on the use of VA-ECMO for the treatment of children with β-blocker poisoning were identified; thus, the pediatric recommendation for children is extrapolated from the adult data.
6. and 7. The Extracorporeal Treatments in Poisoning workgroup based on case reports and mechanistic studies of adults with impaired kidney function provides weak recommendations for hemodialysis to manage critical poisoning due to atenolol or sotalol.24 Nadolol is also considered dialysable, but clinical data are lacking.24 No pediatric data on the use of hemodialysis for the treatment of children with β-blocker poisoning were identified; thus, the recommendation for children is extrapolated from the adult data.
8. Survival after ILE therapy (Table 4) has been described in adult case reports and case series with patients with β-blocker poisoning.25-27 One case of a 7-month-old female did not have improved hemodynamics after ILE administration.28 Two adult cases of severe β-blocker poisoning, combined with bupropion in 1 case and diltiazem in another, reported abrupt cardiac arrest immediately after ILE administration.29 An expert consensus recommendation by international medical toxicology collaborators advises against the routine use of ILE for β-blocker poisoning.25 Given pediatric data on the use of ILE therapy are limited, the recommendation for children is extrapolated from the adult data.
9. Survival after IV calcium administration (Table 4) has been described in adult case reports of cardiac arrest after β-blocker overdose.30,31 These cases are confounded by the concurrent administration of other vasoactive medications, and data for isolated calcium administration is lacking.30,31 No pediatric data on the use of calcium for β-blocker poisoning were identified, thus, recommendations for children are extrapolated from the adult case reports.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. High-dose insulin should be administered for hypotension in adults and children with life-threatening CCB poisoning. |
| 1 | C-LD | 2. Vasopressors should be administered for hypotension in adults and children with life-threatening CCB poisoning. |
| 2a | B-NR | 3. It is reasonable to use ECLS in adults with CCB poisoning that is refractory to pharmacological interventions. |
| 2a | C-LD | 4. It is reasonable to use ECLS in children with CCB poisoning that is refractory to pharmacological interventions. |
| 2a | C-EO | 5. It is reasonable to administer calcium in adults and children with life-threatening CCB poisoning. |
| 2b | C-EO | 6. The usefulness of a glucagon bolus followed by continuous infusion for adults and children with life-threatening CCB poisoning is uncertain. |
| 2b | C-EO | 7. The usefulness of methylene blue for adults and children with refractory vasodilatory shock due to CCB poisoning is uncertain. |
| 2b | C-EO | 8. The usefulness of ILE for adults and children with refractory shock due to CCB poisoning is uncertain. |
Synopsis
Antagonists of the L-type calcium channel (commonly called CCBs) are divided into 2 pharmacological classes: dihydropyridines (eg, nifedipine, amlodipine) and nondihydropyridines (eg, diltiazem, verapamil). At therapeutic doses, nondihydropyridines have more pronounced effects on cardiac tissue, including the atrioventricular node, resulting in negative chronotropy. In contrast, dihydropyridines primarily cause peripheral vasodilation. Amlodipine can cause profound vasodilation mediated by nitric oxide release.1 These distinctions are often lost when therapeutic doses are exceeded, and patients present with severe shock from bradycardia, vasodilation, or loss of inotropy. Prolonged effects are common given that CCBs are frequently prescribed in sustained-release forms (eg, diltiazem, verapamil, nifedipine) or have long half-lives (eg, amlodipine). As a result, CCBs are a leading cause of poisoning mortality reported to poison centers.2 Commonly used treatment modalities include atropine, calcium, vasopressors, high-dose insulin therapy, methylene blue (a nitric oxide synthase inhibitor), and ILE therapy. ECLS, such as VA-ECMO, has been used in refractory cases.
These recommendations inform the management of adults and children with life-threatening CCB poisoning, including cardiac arrest.
Recommendation-Specific Supportive Text
1. High-dose insulin (Table 4) improves inotropy in adults and children with severe cardiogenic shock from CCB poisoning.3-6 One large observational study including adults and children reported favorable outcomes with lower rates of vasoconstrictive complications than vasopressor-only therapy.3 One retrospective cohort study of adults and children suggested that patients with dihydropyridine toxicity may require more vasopressor support along with high-dose insulin because of additive vasodilation.7 Protocolized care reduces the risk of hypoglycemia.3,5 Hypokalemia and volume overload are additional concerns.3,8
2. Many adults and children with CCB-induced shock reported to a poison center registry and in 1 case series received vasopressor therapy.2,9,10 One retrospective case series of 48 adults and children demonstrated excellent survival rates with the primary use of vasopressors (most commonly norepinephrine at doses up to 100 mcg/min in adults), with low rates of ischemic complications.9 Three patients in this series had cardiac arrest before vasopressor therapy. There is no evidence to guide the choice of vasopressors. Other nonadrenergic vasopressors, such as vasopressin, angiotensin II, amrinone, milrinone, and hydroxocobalamin, are not supported by sufficient evidence for a recommendation.
3. and 4. The use of VA-ECMO for adults and children with refractory cardiogenic shock or cardiac arrest after CCB overdose is described in a case series with reported survival rates as high as 77%.11-16 A study using data from the ELSO Registry of adults with CCB poisoning found a mortality rate of 40.6%, which is lower than the general mortality rate of 50% for all adults on ECMO.17 The study also found that a pH less than 7.1 and the use of kidney replacement therapy prior to initiation of ECLS were associated with increased odds of mortality.17
5. The available literature on calcium monotherapy for severe CCB toxicity is limited (Table 4). Improvements in heart rate, blood pressure, and conduction abnormalities are reported in adults and children.18,19 However, most patients require additional treatments.3,15,18,20 In 1 case series of adults, high doses of calcium gluconate (targeting ionized calcium concentrations up to twice normal) appeared to be more effective than lower doses.18
6. Glucagon is an adjunctive therapy for severe CCB poisoning in adults and children.3,9,20 Reported response rates are variable, vomiting is common, and tachyphylaxis may occur.19,21,22
7. Methylene blue (Table 4), a nitric oxide synthase inhibitor, is described in adult case series and case reports as an effective adjunct to treat refractory vasodilatory shock after CCB overdose (involving primarily amlodipine).23 However, responses are mixed, and the effects may be transient. Data for the treatment of children with methylene blue for CCB poisoning is lacking, and recommendations are extrapolated from adult data.
8. An adult case series and a systematic review of adult and pediatric case reports with CCB poisoning identified cases where ILE appeared beneficial.24-26 In 1 report of 2 adults, the use of ILE was associated with abrupt cardiac arrest.8 An expert consensus recommendation by international medical toxicology collaborators advises against the routine use of ILE for CCB poisoning.24 The role of ILE in patients who have failed other modalities and are in cardiac arrest or peri-arrest is unclear.21,24 Data for the treatment of children with ILE for CCB poisoning is limited, and recommendations are extrapolated primarily from adult data.
The clinical manifestations of cocaine poisoning are mainly caused by sympathetic nervous system effects. Cocaine produces a sympathomimetic toxidrome marked by tachycardia, hypertension, hyperthermia, diaphoresis, increased psychomotor activity, and seizures.1 Cocaine induces tachycardia (postsynaptic β-adrenergic receptor agonism) and hypertension (peripheral postsynaptic alpha-adrenergic receptor agonism) by enhanced catecholamine release and catecholamine reuptake inhibition. The release and reuptake blockade of norepinephrine, epinephrine, dopamine, and serotonin also causes the CNS and neuropsychiatric symptoms of cocaine poisoning.1,2 Cocaine-induced coronary atherosclerosis and vasospasm predisposes individuals to acute coronary syndromes.3
The electrocardiographic changes and dysrhythmogenic properties of cocaine are a result of the effect of cocaine on cardiac sodium and potassium channels.4 Sodium channel blockade leads to slowed conductance during phase 0 of the cardiac action potential. As a result, patients may develop QRS complex prolongation and wide-complex tachycardia similar to what occurs with Vaughan-Williams Ia and Ic medications.5,6 Cocaine also causes QT interval prolongation and torsade de pointes through potassium channel blockade.1,7 Cocaine-induced arrhythmias include asystolic cardiac arrest and pulseless VT. Like other local anesthetics, cocaine blocks neuronal sodium channels.
These recommendations inform the management of adults and children with life-threatening cocaine poisoning, including cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. Sedation is recommended for adults with severe agitation from cocaine poisoning. |
| 1 | C-LD | 2. Rapid external cooling with ice water immersion is recommended for adults with life-threatening hyperthermia (core temperature greater than 40 °C [104 °F]) from cocaine poisoning. |
| 2a | C-LD | 3. In adults with wide-complex tachycardia or cardiac arrest from cocaine poisoning, it is reasonable to administer sodium bicarbonate. |
| 2a | C-LD | 4. In adults with wide-complex tachycardia or cardiac arrest from cocaine poisoning, it is reasonable to administer lidocaine. |
| 2a | C-LD | 5. In adults with cocaine-induced coronary vasospasm or hypertensive emergencies, it is reasonable to administer vasodilators (eg, nitrates, phentolamine, CCBs). |
| 2a | C-EO | 6. It is reasonable to use ECLS in adults with cardiogenic shock from cocaine poisoning refractory to other treatment measures. |
| 3: No Benefit | C-LD | 7. In adults with cocaine-induced coronary vasospasm or hypertensive emergencies, administration of β-adrenergic antagonists is not recommended. |
| 3: Harm | C-LD | 8. Prolonged use of physical restraint in adults with life-threatening cocaine poisoning without sedation is potentially harmful. |
Adults with Cocaine Poisoning
Recommendation-Specific Supportive Text
- Sedatives (eg, benzodiazepines and antipsychotics) have been successful in treating adults with cocaine-induced agitation.8 Small animal pretreatment and rescue studies comparing chlorpromazine to diazepam demonstrated improved survival with both compared with controls.9,10 One adult randomized clinical trial comparing droperidol versus lorazepam for acutely agitated patients from cocaine or methamphetamine intoxication found adequate sedation with both medications, but droperidol had faster onset of sedation and required fewer repeat doses.11
- Hyperthermia (core temperature greater than 40 °C [104 °F]) can be rapidly life-threatening in cocaine poisoning.12,13 High-quality data on cooling measures for hyperthermic cocaine-intoxicated adults are lacking, but immersion seems effective.14 Evaporative or immersive cooling modalities reduce temperature more rapidly in adults than cooling blankets, the application of cold packs, or endovascular cooling devices.12,15-21 Ice water immersion resulted in the fastest cooling rates in adults and may cool faster than 0.15 °C/min (0.27 °F/min), which has been associated with increased survival (refer to the Hyperthermia section).16,22
- Canine studies demonstrate the ability of sodium bicarbonate (Table 4) to reverse cocaine-induced QRS prolongation through competitive binding between sodium bicarbonate and cocaine at the sodium channel.23-25 Adult case reports and case series describe the successful use of hypertonic sodium bicarbonate to treat wide-complex tachycardia from severe cocaine poisoning.26-30 The successful use of hypertonic sodium bicarbonate in the resuscitation has also been reported in an adult case report with asystolic cardiac arrest and subsequent wide-complex Brugada pattern.28
- Lidocaine administration has demonstrated safety in a multicenter retrospective observation study of 27 adults with cocaine-induced myocardial infarction.31 The use of lidocaine for cocaine-associated cardiac arrest with ventricular arrhythmias is supported by 1 adult and a pediatric case report in a 17-year-old female patient.32,33
- Adult clinical trials demonstrate improvements in coronary blood flow and myocardial oxygen delivery in patients with cocaine-induced coronary vasospasm after treatment with vasodilators (eg, phentolamine, nitrates, verapamil).34-38 These studies did not include patients with cardiac arrest or in peri-arrest. Adults with refractory ischemia from cocaine were successfully treated with phentolamine.35,39
- ECLS, including VA-ECMO and intra-aortic balloon pump, has been used successfully to support cardiac output in adults in cardiogenic shock after myocardial infarction.40,41 Evidence in patients with cocaine-associated circulatory failure is lacking.
- While a 2008 AHA scientific statement suggests potential benefits of β-adrenergic blockade after acute management of cocaine-related myocardial infarction, evidence to support the use of β-adrenergic blockade in adults with acute life-threatening cocaine toxicity is lacking.42 In controlled animal trials, propranolol failed to prevent cocaine lethality.9,10,43 In adults who received cocaine while undergoing cardiac catheterization, propranolol exacerbated coronary vasoconstriction.44 In the same human model, labetalol was no better than saline at reversing coronary vasoconstriction.45 In at least 1 case report, the administration of metoprolol to an adult with persistent cocaine-induced hypertension and tachycardia resulted in crushing substernal chest pain, which was followed by PEA and death.46 In a case series of adults with acute cocaine toxicity, the effect of esmolol was inconsistent, failing to treat tachycardia in 1 patient and raising diastolic blood pressure (possibly an unopposed alpha-adrenergic effect) in others, 1 of which was severe.47
- Although physical restraints may be necessary temporarily, their sustained use without effective sedation is associated with death in adults with severe agitation from exposure to cocaine and other sympathomimetics.48,49
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Sedation is recommended for children with severe agitation from cocaine poisoning. |
| 1 | C-EO | 2. Rapid external cooling with ice water immersion is recommended for children with life-threatening hyperthermia (core temperature greater than 40 °C [104 °F]) from cocaine poisoning. |
| 2a | C-EO | 3. In children with wide-complex tachycardia or cardiac arrest from cocaine poisoning, it is reasonable to administer sodium bicarbonate. |
| 2a | C-EO | 4. In children with wide-complex tachycardia or cardiac arrest from cocaine poisoning, it is reasonable to administer lidocaine. |
| 2a | C-EO | 5. In children with cocaine-induced coronary vasospasm or hypertensive emergencies, it is reasonable to administer vasodilators (eg, nitrates, phentolamine, CCBs). |
| 2a | C-EO | 6. It is reasonable to use ECLS in children with cardiogenic shock from cocaine poisoning refractory to other treatment measures. |
| 3: No Benefit | C-EO | 7. In children with cocaine-induced coronary vasospasm or hypertensive emergencies, administration of β-adrenergic antagonists should not be performed. |
| 3: Harm | C-EO | 8. Prolonged use of physical restraint in children with life-threatening cocaine poisoning without sedation is potentially harmful. |
Children with Cocaine Poisoning
Recommendation-Specific Supportive Text
- Sedatives (eg, benzodiazepines and antipsychotics) have been successful in treating adults with cocaine-induced agitation.8 Small animal pretreatment and rescue studies comparing chlorpromazine, an antipsychotic, to diazepam, a benzodiazepine, demonstrated improved survival with both compared with controls.9,10 One adult randomized clinical trial comparing droperidol versus lorazepam for acutely agitated patients with cocaine or methamphetamine intoxication found adequate sedation with both medications, but droperidol had faster onset of sedation and required fewer repeat doses.11 No pediatric data on the use of sedatives for life-threatening cocaine poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
- Hyperthermia (core temperature >40 °C [104 °F]) can be rapidly life-threatening in cocaine poisoning.12,13 Evaporative or immersive cooling modalities reduce temperature more rapidly in adults than cooling blankets, the application of cold packs, or endovascular cooling devices.12,15-21 Ice water immersion resulted in the fastest cooling rates in adults and critically may cool faster than 0.15 °C/min (0.27 °F/min), which has been associated with increased survival.16 High-quality data on cooling measures for hyperthermic cocaine-intoxicated adults are lacking, but immersion seems effective.14 No pediatric data on the use of rapid external cooling for life-threatening cocaine poisoning were identified, thus, the recommendation for children is extrapolated from the adult data (refer to the Hyperthermia section).
- Canine studies demonstrate the ability of sodium bicarbonate (Table 4) to reverse cocaine-induced QRS prolongation through competitive binding between sodium bicarbonate and cocaine at the sodium channel.23-25 Adult case reports and case series describe the successful use of hypertonic sodium bicarbonate to treat wide-complex tachycardia from severe cocaine poisoning.26-30 The successful use of hypertonic sodium bicarbonate in resuscitation has also been reported in an adult with asystolic cardiac arrest and subsequent wide-complex Brugada pattern.28 No pediatric data on the use of hypertonic sodium bicarbonate for wide-complex tachycardia or cardiac arrest from cocaine poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
- Lidocaine administration has demonstrated safety in a multicenter retrospective observation study of 27 adults with cocaine-induced myocardial infarction.31 The use of lidocaine for cocaine-associated cardiac arrest with ventricular arrhythmias is supported by 1 adult and a pediatric case report in a 17-year-old female patient.32,33 No other pediatric data on the use of lidocaine for wide-complex tachycardia or cardiac arrest from cocaine poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
- Adult clinical trials demonstrate improvements in coronary blood flow and myocardial oxygen delivery in patients with cocaine-induced coronary vasospasm after treatment with vasodilators (eg, phentolamine, nitrates, verapamil).34,37,38 These studies did not include patients with cardiac arrest or peri-arrest states. Adults with refractory ischemia from cocaine were successfully treated with phentolamine.35,39 No pediatric data on the use of vasodilators for cocaine-induced coronary vasospasm or hypertensive emergencies were identified, thus, the recommendation for children is extrapolated from the adult data.
- ECLS, including VA-ECMO and intra-aortic balloon pump, has been used successfully to support cardiac output in adults in cardiogenic shock after myocardial infarction.40,41 Evidence in patients with cocaine-associated circulatory failure is lacking. No pediatric data on the use of ECLS for cardiogenic shock from cocaine poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
- While a 2008 AHA scientific statement suggests potential benefits of β-adrenergic blockade after acute management of cocaine-related myocardial infarction, evidence to support the use of β-adrenergic blockade in adults with acute life-threatening cocaine toxicity is lacking.42 In controlled animal trials, propranolol and labetalol failed to prevent cocaine lethality. In adults who receive cocaine while undergoing cardiac catheterization, propranolol exacerbates coronary vasoconstriction.9,10,43,44 In the same human model, labetalol was no better than saline at reversing coronary vasoconstriction.45 In at least 1 case report, the administration of metoprolol to an adult with persistent cocaine-induced hypertension and tachycardia resulted in crushing substernal chest pain, which was followed by PEA and death. In a case series of adults with acute cocaine toxicity, the effect of esmolol was inconsistent, failing to treat tachycardia in 1 patient and raising diastolic blood pressure (possibly an unopposed alpha-adrenergic effect) in others, 1 of which was severe.47 No pediatric data on the use of β-adrenergic blockade for cocaine-induced coronary vasospasm or hypertensive emergencies were identified, thus, the recommendation for children is extrapolated from the adult data.
- Although physical restraints may be necessary temporarily, their sustained use without effective sedation is associated with death in adults with severe agitation from exposure to cocaine and other sympathomimetics.48,49 No pediatric data on the use of restraints for life-threatening cocaine poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
Cyanide may be encountered through multiple industrial sources, including jewelry cleaning and electroplating or by the metabolism of naturally occurring cyanogens found in the pits of stone fruits, such as apricots. Cyanide gas is liberated by the incomplete combustion of nitrogen-containing products, such as plastics and vinyl, during house fires. It may also be ingested as cyanide salts in poisonings.
Cyanide inhibits cellular respiration in the mitochondria, causing cardiovascular collapse, metabolic acidosis with elevated plasma lactate concentrations, depressed mental status, seizures, and death.1 Confirmatory testing is not rapidly available, and thus, diagnosis and management are dictated by clinical presentation.
Hydroxocobalamin (vitamin B12a) scavenges cyanide on an equimolar basis to form nontoxic cyanocobalamin. Hydroxocobalamin has a rapid onset of action, is simple to use, and is adequate when given alone for cyanide poisoning.2 Administration of hydroxocobalamin leads to deep red coloration of plasma and urine, which may interfere with laboratory analyses and erroneously trigger hemodialysis “blood leak” detectors.2
Alternatively, sodium nitrite and sodium thiosulfate have also been used to treat cyanide poisoning. Sodium nitrite oxidizes hemoglobin to methemoglobin, which then binds cyanide to form cyanmethemoglobin.3,4 Sodium thiosulfate acts as a substrate for cyanide metabolism, slowly forming minimally toxic thiocyanate.3
These recommendations inform the management of adults and children with life-threatening cyanide poisoning, including cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Hydroxocobalamin should be administered to adults with life-threatening cyanide poisoning. |
| 1 | C-LD | 2. The combination of sodium nitrite and sodium thiosulfate should be administered to adults with life-threatening cyanide poisoning when hydroxocobalamin is unavailable. |
| 2a | C-EO | 3. In adults with concurrent carbon monoxide and cyanide poisoning (eg, from a house fire), administration of sodium thiosulfate alone is reasonable when hydroxocobalamin is unavailable. |
| 2b | C-EO | 4. In addition to administering hydroxocobalamin, it may be reasonable to administer sodium thiosulfate to adults with cyanide poisoning. |
Adults with Cyanide Poisoning
Recommendation-Specific Supportive Text
- Hydroxocobalamin (Table 4) has been demonstrated to improve the survival of patients with cyanide poisoning. One prospective observational case series included 69 adults with suspected cyanide poisoning from house fires who were treated with hydroxocobalamin.5 Fifty (72%) of the patients survived. Fifteen patients received hydroxocobalamin while in cardiac arrest, and only 2 (13%) survived.5 In a case series of 13 adults and a 14-year-old patient with cyanide poisoning not associated with house fires, 10 (10/14 71%) patients survived, including 7 (7/9 78%) patients who were treated with hydroxocobalamin alone.6 The 4 fatalities in this review were all patients who were in cardiac arrest when they received hydroxocobalamin.6 Overall, mortality of patients in cardiac arrest from cyanide poisoning is high despite hydroxocobalamin administration, highlighting the severity of disease. Adverse effects of hydroxocobalamin include transient hypertension, skin discoloration, rash, acute kidney injury, interference with colorimetric laboratory assays, and erroneously triggering of hemodialysis "blood leak" detectors.5-9,18 Limited pediatric data on the treatment of life-threatening cyanide poisoning with hydroxocobalamin were identified, thus, the recommendation for children is extrapolated primarily from the adult data.
- The combination of sodium nitrite and sodium thiosulfate was more protective against lethality in a canine model than either sodium nitrite or sodium thiosulfate alone.10 Adult cases of life-threatening cyanide poisoning have demonstrated survival using the sodium nitrite and sodium thiosulfate combination (Table 4).11 To avoid excessive methemoglobin formation, the dosing of sodium nitrite in children or in patients with anemia must be precise; the prescribing information lists specifications.12
- Sodium nitrite can produce methemoglobinemia, reduces oxygen-carrying capacity, and may worsen cellular hypoxia in adults with concomitant carbon monoxide poisoning.10 The sodium thiosulfate alone was protective against lethality in a canine model compared with controls.10 A conflicting porcine model found sodium thiosulfate alone did not reverse shock in pigs with cyanide poisoning.13 No human clinical trial exists to inform the use of sodium thiosulfate alone to treat cyanide poisoning. Adults and children in cases of life-threatening cyanide poisoning have survived after treatment with sodium thiosulfate alone (Table 4).14,15
- Survival after hydroxocobalamin with sodium thiosulfate to treat cyanide poisoning has been reported in adult cases.16,17 There are no human trials comparing the use of hydroxocobalamin alone versus in combination with sodium thiosulfate. One porcine study showed no difference in mean arterial pressures or survival when sodium thiosulfate was administered in combination with hydroxocobalamin versus hydroxocobalamin alone.13
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Hydroxocobalamin should be administered to children with life-threatening cyanide poisoning. |
| 1 | C-EO | 2. The combination of sodium nitrite and sodium thiosulfate should be administered to children with life-threatening cyanide poisoning when hydroxocobalamin is unavailable. |
| 2a | C-EO | 3. In children with concurrent carbon monoxide and cyanide toxicity (eg, from a house fire), administration of sodium thiosulfate alone is reasonable when hydroxocobalamin is unavailable. |
| 2b | C-EO | 4. In addition to administering hydroxocobalamin, it may be reasonable to administer sodium thiosulfate to children with cyanide poisoning. |
Children with Cyanide Poisoning
Recommendation-Specific Supportive Text
- Hydroxocobalamin (Table 4) has been demonstrated to improve the survival of patients with cyanide poisoning. One prospective observational case series included 69 adults with suspected cyanide poisoning from house fires who were treated with hydroxocobalamin.5 Fifty (72%) of the patients survived. Fifteen patients received hydroxocobalamin while in cardiac arrest, and only 2 (13%) survived.5 In a case series of 13 adults and a 14-year-old patient with cyanide poisoning not associated with house fires, 10 (10/14; 71%) patients survived, including 7 (7/9; 78%) patients who were treated with hydroxocobalamin alone.6 The 4 fatalities in this review were all patients who were in cardiac arrest when they received hydroxocobalamin.6 Overall, mortality of patients in cardiac arrest from cyanide poisoning is high despite hydroxocobalamin administration, highlighting the severity of disease. Adverse effects of hydroxocobalamin include transient hypertension, skin discoloration, rash, acute kidney injury, interference with colorimetric laboratory assays, and erroneously triggering of hemodialysis “blood leak” detectors.5-9,18 Limited pediatric data on the treatment of life-threatening cyanide poisoning with hydroxocobalamin were identified, thus, the recommendation for children is extrapolated primarily from the adult data.
- The combination of sodium nitrite and sodium thiosulfate was more protective against lethality in a canine model than either sodium nitrite or sodium thiosulfate alone.10 Adult cases of life-threatening cyanide poisoning have demonstrated survival using the sodium nitrite and sodium thiosulfate combination.11 To avoid excessive methemoglobin formation, the dosing of sodium nitrite in children or in patients with anemia must be precise; the prescribing information lists specifications.12 No pediatric data on the treatment of life-threatening cyanide poisoning with the combination sodium nitrite and sodium thiosulfate were identified, thus, the recommendation for children is extrapolated from the adult data (Table 4).
- Sodium nitrite can produce methemoglobinemia, reduces oxygen-carrying capacity, and may worsen cellular hypoxia in adults with concomitant carbon monoxide poisoning.10 The sodium thiosulfate alone was protective against lethality in a canine model compared with controls.10 A conflicting porcine model found sodium thiosulfate alone did not reverse shock in pigs with cyanide poisoning.13 No human clinical trial exists to inform the use of sodium thiosulfate alone to treat cyanide poisoning. Adults and children in cases of life-threatening cyanide poisoning have survived after treatment with sodium thiosulfate alone (Table 4).14,15
- Survival after hydroxocobalamin with sodium thiosulfate to treat cyanide poisoning has been reported in adult cases.16,17 There are no human trials comparing the use of hydroxocobalamin alone versus in combination with sodium thiosulfate. One porcine study showed no difference in mean arterial pressures or survival when sodium thiosulfate was administered in combination with hydroxocobalamin versus hydroxocobalamin alone.13 No pediatric data on the treatment of life-threatening cyanide poisoning with the combination of hydroxocobalamin with sodium thiosulfate were identified, thus, the recommendation for children is extrapolated from the adult data (Table 4).
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Digoxin-specific antibody fragments (digoxin-Fab) should be administered for adults and children with life-threatening digoxin or digitoxin poisoning. |
| 2a | C-LD | 2. It is reasonable to administer digoxin-Fab for adults and children with life-threatening poisoning due to Bufo toad and yellow oleander toxins. |
| 2b | C-EO | 3. It may be reasonable to administer digoxin-Fab to treat adults and children with life-threatening poisoning from cardiac glycosides other than digoxin, digitoxin, Bufo toad toxins, and yellow oleander toxins. |
| 2b | C-EO | 4. It may be reasonable to administer lidocaine or phenytoin to treat adults and children with ventricular arrhythmias caused by digoxin and related cardiac glycoside poisoning until digoxin-Fab can be administered. |
| 3: No Benefit | B-NR | 5. The use of hemodialysis, hemofiltration, hemoperfusion, or plasmapheresis to treat adults and children with life-threatening poisoning from digoxin and related cardiac glycosides is not recommended. |
Synopsis
Cardiac glycoside poisoning can be caused by medications such as digoxin and digitoxin, plants such as foxglove and oleander, and certain toad venoms ingested as ethnopharmaceuticals or hallucinogens. Patients with cardiac glycoside poisoning may develop gastrointestinal symptoms, confusion, hyperkalemia, and cardiac conduction abnormalities, including atrioventricular nodal block, VT, VF, or asystole. Although cardiac glycosides include a range of structurally similar cardioactive steroids, most data involve digoxin poisoning.
Digoxin-specific immune antibody fragments (eg, digoxin-Fab) bind to and inactivate digoxin and structurally similar cardiac glycosides. Different dosing regimens are advocated worldwide.1-3 The ideal empirical dose for cardiac arrest is unknown and likely differs for digoxin poisoning compared with other cardiac glycosides.
Standard ALS measures for bradycardia, such as atropine and electrical pacing, have variable effectiveness in cardiac glycoside poisoning. Prior recommendations addressing these interventions were removed since they are included as part of standard ALS measures.
These recommendations inform the management of adults and children with life-threatening poisoning from digoxin and related cardiac glycosides, including cardiac arrest.
Recommendation-Specific Supportive Text
- A systematic review of digoxin poisoning in adults and children found observational studies showing resolution of life-threatening arrhythmias after digoxin-Fab administration (Table 4).2 Most studies report response rates of 50% to 90%, with arrhythmia resolution in 30 to 45 minutes in most cases.2 Although there are no RCTs studying cardiac arrest from digoxin or digitoxin poisoning, excellent survival (30/56 [54%] patients) was reported in an observational study of adults and children in cardiac arrest treated with digoxin-Fab.4
- An RCT among hemodynamically stable adults and children with yellow oleander (Thevetia peruviana, also known as Cascabela thevetia) cardiotoxicity (bradycardia or atrial tachyarrhythmias) showed significant resolution of arrhythmia with digoxin-Fab, as do many case reports and case series including adults and children (Table 4).5,6 A Cochrane review did not identify any trials in adults or children with severe yellow oleander toxicity.7 In vitro studies showed affinity between cardiac glycosides found in Bufo toad toxin and digoxin-Fab, murine studies showed protection, and some published case reports of adults and children showed apparent response.8-14
- Survival after the administration of digoxin-Fab for poisoning from cardiac glycosides other than digoxin, digitoxin, Bufo toad toxin, and yellow oleander are limited to case reports in adults only.5,15-21 No pediatric data on the use of digoxin-Fab to treat poisoning from cardiac glycosides other than digoxin, digitoxin, Bufo toad toxin, and yellow oleander were identified, thus, the recommendation for children is extrapolated from the adult data (Table 4).
- There are multiple case reports including adults and children describing the use of antidysrhythmic medications, including lidocaine and phenytoin, to treat ventricular arrhythmias caused by digoxin poisoning, with various responses.22-24 No high-quality cohort studies or randomized trials have evaluated their effect.
- A systematic review of adults and children receiving extracorporeal treatment for digoxin poisoning found that digoxin is not well removed because of its large volume of distribution.25
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. For adults and children with life-threatening local anesthetic poisoning, ILE should be administered. |
| 1 | C-LD | 2. Benzodiazepines should be used to treat adults and children with seizures associated with local anesthetic systemic toxicity (LAST). |
| 2a | C-EO | 3. It is reasonable to administer sodium bicarbonate to adults and children for life-threatening wide complex tachycardia associated with local anesthetic toxicity. |
| 2a | C-EO | 4. It is reasonable to use ECLS in adults and children with local anesthetic toxicity with refractory cardiogenic shock. |
Synopsis
Local anesthetics reversibly bind sodium channels to disrupt nerve transmission and block pain signals. Patients with local anesthetic poisoning present with a constellation of CNS and cardiovascular symptoms termed local anesthetic systemic toxicity (LAST). CNS toxicity includes agitation, confusion, obtundation, and seizures, whereas cardiovascular symptoms include asystole, bradycardia, cardiac collapse, conduction delays, hypertension, hypotension, and ventricular arrhythmias.1-3 Cardiovascular and CNS toxicity can occur both in isolation and concomitantly.3-5
Local anesthetics vary in toxicity depending on the potency associated with their lipophilic side chains. At equal doses (eg, mg/kg), bupivacaine is a more potent cardiotoxin than lidocaine and ropivacaine through its greater affinity and binding durations to cardiac sodium channels.6,7 However, at equipotent doses, all local anesthetics can precipitate toxicity.2-4,8
Both hypoxia and acidemia are associated with increased toxicity from bupivacaine in animal models.9-11 As such, airway management and circulatory support are critical to resuscitation from LAST. Pharmacological interventions (eg, ILE, sodium bicarbonate) and mechanical support (eg, VA-ECMO) in addition to ACLS have been used for LAST.
Evidence-based dosing recommendations for ILE are lacking. The majority of animal studies and human experience for the treatment of LAST use 20% ILE.9 Attempts to reproduce this dose using propofol (which contains 10 mg/mL propofol in 10% ILE) would be at 10 to 20 times the induction dose for general anesthesia and lead to serious CNS and cardiovascular adverse effects.
Local anesthetic poisoning can also produce methemoglobinemia; treatment recommendations are provided in the Toxicology: Methemoglobinemia section.
These recommendations inform the management of adults and children with life-threatening local anesthetic poisoning, including cardiac arrest.
Recommendation-Specific Supportive Text
- Early administration of 20% ILE (Table 4) in patients with LAST is supported by animal studies, case reports (including in children and pregnant women), and registry studies.1,4,9,12-19 In conjunction with prevention of hypoxia and acidemia through standard ALS measures, administration of ILE has been associated with successful resuscitation in these studies. The descriptive studies are subject to publication bias with only a few case reports where ILE failed to resuscitate patients with LAST associated with lidocaine, mepivacaine, and ropivacaine.20-23 Three RCTs (n=40 volunteers) demonstrate a potential pharmacokinetic benefit of ILE for ropivacaine, levobupivacaine, bupivacaine, and lidocaine. These 3 studies found that coadministration of ILE decreased the maximum plasma concentration of ropivacaine, the concentration curve for lidocaine, and the context-sensitive half-life of bupivacaine.12,24,25 This is consistent with animal models of the same phenomenon.26,27
- In the case of isolated CNS toxicity, LAST may progress rapidly to cardiovascular toxicity. Seizures associated with LAST may worsen hypoxia and acidemia, increasing the risk of cardiotoxicity.11 Administration of benzodiazepines can abort seizure-like activity and is commonly reported as part of a therapeutic regimen in adults and children.2,15,28
- Sodium bicarbonate administration (Table 4) may overcome sodium channel blockade by local anesthetics and correct acidemia. Evidence to support the use of sodium bicarbonate is limited to case reports in adults and children as part of a therapeutic regimen.15,28 However, some case reports showed limited efficacy in adults already in cardiac arrest.29,30 There are multiple porcine RCTs demonstrating effective shortening of the QRS interval in bupivacaine and ropivacaine toxicity.31,32
- Several case reports in adults and children describe successful use of mechanical circulatory support, such as CPB or VA-ECMO, for patients with LAST and refractory cardiogenic shock.29,30,33-36 Unfortunately, lack of widespread availability of VA-ECMO limits use of these interventions.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For adults and children with life‐threatening methemoglobinemia, methylene blue should be administered. |
| 2b | C-EO | 2. Exchange transfusion may be reasonable as a treatment for adults and children with life-threatening methemoglobinemia that is not responsive to methylene blue. |
| 2b | C-EO | 3. Hyperbaric oxygen therapy may be reasonable as a treatment for adults and children with life-threatening methemoglobinemia that is not responsive to methylene blue. |
| 2b | C-EO | 4. For adults and children with life-threatening methemoglobinemia, it may be reasonable to administer ascorbic acid only when methylene blue is contraindicated or not available. |
| 3: No Benefit | B-R | 5. N-acetylcysteine is not recommended as a treatment for adults with life-threatening methemoglobinemia. |
Synopsis
Acquired methemoglobinemia occurs after exposure to an oxidant stressor that oxidizes iron in the hemoglobin molecule from the ferrous (Fe2+) state to the ferric (Fe3+) state. In the ferric state, hemoglobin no longer effectively binds and delivers oxygen to end organs. Common sources of oxidant stress that can cause methemoglobinemia include nitrates, nitrites, and many pharmaceuticals (eg, benzocaine, dapsone, phenazopyridine).1-9 Patients with methemoglobinemia can appear cyanotic and dusky and complain of shortness of breath and fatigue. Frequently, a difference is observed between the oxygen saturation measured by pulse oximetry versus arterial blood gas. Although moderate methemoglobinemia is generally well-tolerated, severe methemoglobinemia can lead to cardiovascular collapse and death.5,6,9
The most widely accepted treatment for methemoglobinemia is methylene blue, which acts as a cofactor to reduce methemoglobin to hemoglobin.10 Other treatment modalities that have been described include exchange transfusion, hyperbaric oxygen therapy, and ascorbic acid. No studies have examined the treatment of methemoglobinemia in the context of cardiac arrest.
These recommendations inform the management of adults and children with life-threatening methemoglobinemia, including cardiac arrest.
Recommendation-Specific Supportive Text
- No randomized trial has evaluated methylene blue for the treatment for methemoglobinemia; however, observational studies and published case reports in adults and children consistently demonstrate that methylene blue effectively reverses methemoglobinemia (Table 4).1-4,7,11-13 Methylene blue may not improve methemoglobinemia and may lead to hemolysis in patients who have glucose-6-phosphate dehydrogenase deficiency, which is present in about 2% of the US population.13-17
- Exchange transfusion has been used successfully in case reports and case series in adults and children to treat methemoglobinemia and may be appropriate in patients for whom methylene blue is ineffective.18-24
- Hyperbaric oxygen therapy has been used as monotherapy and in conjunction with other therapies in adults. Reduction of methemoglobinemia concentrations, however, can be delayed up to several hours.25-27 Not all health care facilities will have hyperbaric oxygen therapy available, and its use may be impractical in the setting of cardiopulmonary collapse or cardiac arrest. There are no published pediatric cases of the use of hyperbaric oxygen therapy for methemoglobinemia. Recommendations for children are extrapolated from adult case reports.
- Ascorbic acid, or vitamin C, has been used to treat methemoglobinemia.23,28-32 Most published case reports in adults and children demonstrate its use in conjunction with other treatment modalities or as an alternative when methylene blue is contraindicated or unavailable. The clinical effect of ascorbic acid, however, is slow, often requires multiple doses over several hours, and might require higher doses to achieve similar reductions on methemoglobin.5,28-30,32-34 It is, therefore, unlikely to have benefit when used alone in resuscitation situations.
- N-acetylcysteine did not reduce sodium nitrite–induced methemoglobinemia in a double-blind crossover adult volunteer study.35 No pediatric data on the use of N-acetylcysteine were identified.
Opioid poisoning continues in the United States and other countries at epidemic levels.1 Most opioid overdose deaths are unintentional, which underscores the need to develop and implement evidence-based interventions for primary prevention, emergency treatment, and recovery support for people with substance use disorder. Response to life-threatening opioid overdose is a critical resuscitation skill.
In formulating these recommendations, the writing group reviewed prior AHA publications about opioid overdose resuscitation and a 2024 ILCOR systematic review and performed a structured literature review through November 2024.2-9 We acknowledge the contributions of the authors of prior AHA opioid overdose recommendations, members of the 2025 Adult Basic Life Support Writing Group, and Dr. Matthew Neth for their contributions to the Toxicology: Opioid Overdose section.2-4,6-9
Recommendations about training of the lay rescuers for opioid overdose recognition and response are provided in "Part 12: Resuscitation Education Science." The treatment of opioid-induced respiratory depression in newborn infants is outside the scope of these guidelines.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For trained rescuers assisting an adult or child with suspected opioid overdose who has respiratory depression or respiratory arrest and has a definite pulse, breaths or bag-mask ventilation should be provided. |
| 1 | B-NR | 2. For lay rescuers assisting an adult or child with suspected opioid overdose who is unresponsive and not breathing normally, CPR with breaths should be provided. |
| 1 | B-NR | 3. For trained rescuers assisting an adult or child with suspected opioid overdose in respiratory arrest with a definite pulse, an opioid antagonist (eg, naloxone) should be administered. |
| 1 | C-EO | 4. Lay and trained rescuers should not delay performing standard resuscitation or activating the emergency response system while administering an opioid antagonist (eg, naloxone) or awaiting the person’s response to an opioid antagonist. |
| 2b | B-NR | 5. For lay and trained rescuers, opioid antagonist administration may be reasonable for adults in cardiac arrest with suspected opioid overdose, provided that opioid antagonist (eg, naloxone) administration does not interfere with the delivery of standard resuscitation including high-quality compression-ventilation CPR. |
| 2b | C-EO | 6. For lay and trained rescuers, opioid antagonist administration may be reasonable for children in cardiac arrest with suspected opioid overdose, provided that opioid antagonist (eg, naloxone) administration does not interfere with the delivery of standard resuscitation including high-quality compression-ventilation CPR. |
Adults and Children with Opioid Overdose
Synopsis
Opioid overdose causes CNS and respiratory depression, progressing to respiratory arrest and ultimately cardiac arrest.4 Most opioid deaths involve additional substances that contribute to respiratory depression.10-15 Differentiating opioid overdose from other causes of cardiac and respiratory arrest can be difficult without an accurate history, which is often unavailable.16 The mainstay of care remains early recognition, activation of the emergency response system, provision of breaths or ventilations, and the administration of opioid antagonists for adults and children in respiratory arrest (Figures 7 and 8).
Opioid antagonists can restore spontaneous respirations and protective airway reflexes in adults and children with respiratory depression or respiratory arrest from opioid overdose. For adults with cardiac arrest from opioid overdose, observational studies of naloxone administration have mixed results.5
Two opioid antagonists, naloxone and nalmefene, are currently available for reversal in the United States.17 Both are high potency antagonists of the mu-opioid receptor. Naloxone is currently available for purchase with or without a prescription in the United States and Canada.18,19 Intranasal nalmefene is available by prescription only.20 To date, the published clinical data are more robust for naloxone than nalmefene.
These recommendations inform the management of adults and children with life-threatening opioid poisoning, including respiratory depression, respiratory arrest, and cardiac arrest.
Recommendation-Specific Supportive Text
1, 2, and 4. Initial management of opioid overdose should focus on support of the person’s airway and breathing, as described in "Part 6: Pediatric BLS" and "Part 7: Adult BLS." Respiratory depression and respiratory arrest can be treated by opening the airway and providing breaths or ventilations, which may be all that is needed to prevent deterioration to cardiac arrest. CPR is indicated for any adult or child in known or suspected cardiac arrest, including in trained rescuer situations in which a definite pulse is not felt and in lay rescuer situations in which a pulse check is not performed. Opioid antagonists may require several minutes and repeated doses to reverse respiratory arrest, particularly when administered by the intranasal or IM route.21-23 Nonopioid sedatives commonly contribute to respiratory depression and are typically not reversed by opioid antagonists. Opioid antagonists are extremely unlikely to benefit an adult or child in cardiac arrest who is not receiving CPR and do not reverse VF. For these reasons, standard BLS or ALS resuscitation should be provided immediately and continued until the adult or child is awake and breathing normally. Initiating compression-ventilation CPR before opioid antagonist administration differs from that of the prescribing information for naloxone or nalmefene.19,20 The rationale to initiate standard resuscitation first and continue until the adult or child is breathing normally is articulated in this chapter and in prior AHA publications.3
3. Opioid antagonists restore protective airway reflexes and reverse respiratory arrest from opioid overdose in adults and children. More than 30 observational studies report return of alertness and resolution of respiratory depression in adults and children with opioid overdose when opioid antagonists are administered (for dosing, see Table 4).22-56 No studies compared opioid antagonist administration to standard resuscitation or ventilatory support alone. Major complications are rare and dose related.
5 and 6. No clinical trials have evaluated the role of opioid antagonists in adults or children with cardiac arrest. The results of animal studies and adult observational studies of naloxone administration in undifferentiated cardiac arrest or cardiac arrest with suspected opioid overdose are conflicting and highly susceptible to confounding by indication.57-64 As a result, the overall certainty of the evidence surrounding a potential role for opioid antagonist administration for adults in cardiac arrest is very low.5 However, no human study has clearly demonstrated harm from naloxone administration in cardiac arrest. Pediatric data are sparse, and recommendations for children are extrapolated from adult studies. Identifying opioid overdose as the underlying cause of cardiac arrest may not always be obvious to the rescuer. Furthermore, resuscitation emergencies are task-saturated events. It is important that interventions with proven benefit, such as high-quality compression-ventilation CPR, be prioritized over interventions such as opioid antagonists, which do not have established benefit for adults and children in cardiac arrest and are unlikely to work in the absence of cardiac output.5 For dosing information in adults and children, refer to Table 4.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Adults with opioid overdose who respond to opioid antagonists (eg, naloxone) should be observed in a health care setting until a low risk of recurrent respiratory depression, normal level of consciousness, and normal vital signs are achieved. |
| 1 | C-EO | 2. Children with opioid overdose who respond to opioid antagonists (eg, naloxone) should be observed in a health care setting until a low risk of recurrent respiratory depression, normal level of consciousness, and normal vital signs are achieved. |
| 2a | C-LD | 3. Repeated doses of opioid antagonists (eg, naloxone) can be beneficial in adults with opioid overdose who respond to opioid antagonists and develop recurrent respiratory depression. |
| 2a | C-EO | 4. Repeated doses of opioid antagonists (eg, naloxone) can be beneficial in children with opioid overdose who respond to opioid antagonists and develop recurrent respiratory depression. |
Adults and Children with Opioid Overdose Following Successful Response to Opioid Antagonists
Synopsis
People with opioid overdose who respond to opioid antagonist therapy may develop recurrent respiratory depression if the duration of action of the opioid exceeds the duration of the antagonist.
Recommendation-Specific Supportive Text
1–4. Adults and children with respiratory depression or respiratory arrest who respond to an opioid antagonist may develop recurrent CNS and respiratory depression. In 1 study, recurrent respiratory depression occurred in 72% of patients treated with titrated low doses of IV naloxone.65 The ideal duration of observation is unknown and likely differs based on the substance involved, which may be unknown; the dose and route of opioid antagonist administration; and whether the patient received naloxone or nalmefene. Although abbreviated observation periods may be adequate for adults with fentanyl, morphine, or heroin overdose, a period of observation may be required to safely discharge an adult with a life-threatening overdose of a long-acting or sustained-release opioid.26,49,53,66-74 Treatment options for recurrent respiratory depression include repeat doses of an opioid antagonist or an IV infusion of naloxone (Table 4).49,67,69,74,75 No studies were identified to inform the minimum safe observation period for a child treated with opioid antagonists or the efficacy of opioid antagonists for recurrent respiratory depression in children, thus, recommendations are extrapolated from adult data.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Adults who are treated for opioid overdose should receive an opioid antagonist (eg, naloxone) and teaching at the time of discharge from a health care setting. |
| 1 | C-EO | 2. Children who are treated for opioid overdose, or their caregivers as appropriate, should receive an opioid antagonist (eg, naloxone) and teaching at the time of discharge from a health care setting. |
Opioid Antagonist Education and Distribution
Synopsis
People who survive an opioid overdose are at increased risk of a subsequent overdose. Secondary and tertiary prevention strategies include screening, brief intervention, and referral to treatment (SBIRT); providing or prescribing opioid antagonists such as “take-home naloxone” programs; and prescribing medications for opioid use disorder.76 These recommendations specifically relate to provision or prescribing of naloxone to opioid overdose survivors at the time of discharge from the emergency department or hospital. A 2024 scoping review summarizes 12 studies of programs that distribute (“take-home naloxone”) or prescribe opioid antagonists to patients at the time of discharge from the emergency department or hospital following treatment for opioid overdose.77 Although the programs contained many different components, naloxone distribution was widely accepted by adults and children in these studies. Naloxone kits were used more than 90 000 times over a 4-year period to reverse opioid overdoses in British Columbia.78 In a US emergency medical services–based study, 24 000 adults and children received layperson-administered naloxone over a 2-year period.79 Observational studies show that community-based naloxone distribution programs are associated with lower opioid overdose death rates and are cost-effective.80,81
Recommendation-Specific Supportive Text
1 and 2. Adults and children who survive opioid overdose are at high risk of subsequent fatal overdose.82-85 The highest risk period is the first several days after the initial overdose, and the risk of overdose mortality remains high for at least a year. A 2016 systematic review found that take-home naloxone programs align with the Bradford Hill viewpoints for causality between exposure (opioid antagonist distribution) and outcome (reduced opioid overdose mortality).86 In those studies that reported the age of participants, the majority of participants were adults. Pediatric recommendations are, therefore, extrapolated from adult data.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. Atropine should be administered immediately for adults and children with life-threatening organophosphate or carbamate poisoning. |
| 1 | C-LD | 2. Early endotracheal intubation is recommended for adults with life-threatening organophosphate or carbamate poisoning. |
| 1 | C-EO | 3. Early endotracheal intubation is recommended for children with life-threatening organophosphate or carbamate poisoning. |
| 1 | C-EO | 4. Benzodiazepines should be administered to treat seizures and agitation for adults and children with life-threatening organophosphate or carbamate poisoning. |
| 1 | C-EO | 5. Use of appropriate PPE is recommended when caring for adults and children with life-threatening organophosphate or carbamate exposure. |
| 1 | C-EO | 6. Dermal decontamination for external organophosphate or carbamate exposure is recommended for adults and children with life-threatening organophosphate or carbamate exposure. |
| 2a | A | 7. For adults with life-threatening organophosphate poisoning, the use of pralidoxime is reasonable. |
| 2a | B-R | 8. For children with life-threatening organophosphate poisoning, the use of pralidoxime is reasonable. |
| 3: No Benefit | C-EO | 9. Use of neuromuscular blockers metabolized by cholinesterase (eg, succinylcholine and mivacurium) is not recommended for adults and children with life-threatening organophosphate or carbamate poisoning. |
Synopsis
Organophosphates and carbamates—found in pesticides, nerve agents, and some medications—inhibit acetylcholinesterase, resulting in muscarinic and nicotinic toxicity. They produce parasympathetic excess (bradycardia, bronchorrhea, bronchospasm, diaphoresis, diarrhea, hypersalivation, lacrimation, miosis, urination, vomiting), nicotinic excess (mydriasis, tachycardia, muscle fasciculations progressing to depolarizing neuromuscular blockade and paralysis), and CNS effects (altered mental status, central apnea, seizures).
Early and effective treatment may prevent deterioration to respiratory and cardiac arrest. Treatments include decontamination, atropine, benzodiazepines, and oximes.1
Atropine blocks parasympathetic overstimulation, mitigating bronchorrhea, bradycardia, bronchospasm, and CNS effects. Atropine does not block acetylcholine excess at the neuromuscular junction or nicotinic ganglia and, therefore, does not reverse paralysis. When administered early, oximes reactivate the acetylcholinesterase enzyme, reversing nicotinic effects to slowly improve respiratory and skeletal muscle strength, although this effect may be organophosphate specific.2-5 Hemoperfusion or adsorption for organophosphate poisoning has been evaluated but is not available in North America.6
The evidence for the treatment of cholinesterase poisoning primarily involves adults and children with organophosphate poisoning. The basis for the treatment of patients with life-threatening carbamate poisoning is extrapolated from the limited evidence for carbamate poisoning and indirectly from evidence for organophosphate poisoning.
These recommendations inform the management of adults and children with life-threatening organophosphate or carbamate poisoning, including cardiac arrest.
Recommendation-Specific Supportive Text
1. For adults and children with life-threatening organophosphate or carbamate poisoning including cardiac arrest, bradycardia, hypotension, bronchorrhea, or bronchospasm, early atropine administration improved survival in 1 clinical trial.7 Much larger doses of atropine are often required for this indication than for typical bradycardia (Table 4). The initial dose is doubled every 5 minutes until full atropinization is achieved (clear chest on auscultation; heart rate greater than 80/min; systolic blood pressure greater than 80 mm Hg). Maintenance of atropinization can be achieved by an atropine infusion (Table 4).7
2 and 3. Observational data, mostly in adults, show that patients who are intubated early in the course of organophosphate or carbamate poisoning have shorter durations of intubation than those who are intubated later in their course.9,10 Whether this is due to prevention of aspiration pneumonia, differences in pathophysiology of respiratory failure, or some other cause is not known. Minimal pediatric data on airway management with organophosphate or carbamate poisoning were identified; thus, the recommendation for children is extrapolated from adult data.
4. Benzodiazepines, such as diazepam (first line) or midazolam, have demonstrated efficacy for treating organophosphate or carbamate-induced seizures and agitation in adults and children, and they effectively manage organophosphate-induced status epilepticus and mitigate neuronal injury in animal models.8,11
5. Health care professionals not wearing appropriate PPE have developed symptoms consistent with organophosphate exposure after being in close contact with adults and children poisoned by organophosphates, including patients with respiratory and dermal exposures only.12-15 Appropriate health care professional PPE depends on the circumstances of the organophosphate exposure and potency of the involved organophosphate.
6. Dermal decontamination through removal of contaminated clothing and copious irrigation with soap and water performed by people wearing protective barriers is effective at preventing further absorption and prevents contamination of caregivers and the care environment.1
7 and 8. Metanalysis of 3 RCTs of primarily adults with organophosphate poisoning treated with oximes versus placebo did not find a mortality benefit. The 3 studies were heterogenous, used lower doses of pralidoxime, and all had risks of biases.16 Two high-dose versus low-dose pralidoxime RCTs of primarily adults with moderate to severe organophosphate poisoning favored the high-dose groups with greater survival, lower rates of pneumonia, and lower mean atropine doses (see Table 4 for dosing information).17 These findings are consistent with pralidoxime-induced protection with and without atropine against lethality in a rodent model of organophosphate poisoning.18 Oximes are not universally effective; their effectiveness may be limited by rapid aging of some organophosphates (eg, tabun), their inability to cross the blood-brain barrier, structural differences among organophosphates, and rapid rein activation of regenerated acetylcholinesterase in the presence of poison.4,5 Although the available data are not sufficient to support a recommendation for or against oxime use in carbamate poisoning, oximes should not be withheld in cases of cholinesterase poisoning when the class of poison is unknown. A limited number of children were included in the RCTs of pralidoximes for organophosphate poisoning, thus, the recommendation for children is extrapolated from the adult data.
9. Neuromuscular blockade from medications metabolized by butyrylcholinesterase (pseudocholinesterase), such as succinylcholine and mivacurium, can be prolonged by several hours in the context of organophosphate or carbamate poisoning in adult and pediatric cases.19-21 Other aminosteroid (eg, rocuronium, vecuronium) and benzylisoquinolinium (eg, atracurium, cisatracurium) neuromuscular blockers are not primarily metabolized by cholinesterases.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Sodium bicarbonate should be used to treat adults with life-threatening cardiotoxicity from tricyclic and tetracyclic antidepressant poisoning. |
| 2a | C-LD | 2. It is reasonable to use sodium bicarbonate to treat children with life-threatening cardiotoxicity from tricyclic and tetracyclic antidepressant poisoning. |
| 2a | C-LD | 3. It is reasonable to use hyperventilation in combination with sodium bicarbonate for mechanically ventilated adults and children with life-threatening sodium channel blocker poisoning. |
| 2a | C-EO | 4. It is reasonable to use ECLS in adults and children with refractory cardiogenic shock from life-threatening sodium channel blocker poisoning. |
| 2a | C-EO | 5. It is reasonable to use sodium bicarbonate to treat adults and children with life-threatening cardiotoxicity caused by poisoning from sodium channel blockers other than tricyclic and tetracyclic antidepressants. |
| 2b | C-EO | 6. It may be reasonable to use Vaughan-Williams class Ib antiarrhythmics (eg, lidocaine) to treat adults and children with life-threatening cardiotoxicity from class Ia or Ic sodium channel blockers. |
| 2b | C-EO | 7. It may be reasonable to use ILE to treat adults and children with life-threatening sodium channel blocker poisoning refractory to other treatment modalities. |
Synopsis
Many poisons block cardiac sodium channels with properties similar to Vaughan-Williams class Ia or Ic antiarrhythmics. Sodium channel blocker poisoning causes QRS prolongation, hypotension, seizures, ventricular arrhythmias, and cardiovascular collapse. Characteristic electrocardiogram changes usually precede ventricular arrhythmias in patients with sodium channel blocker poisoning. These include intraventricular conduction delay (QRS complex prolongation) and the development of a terminal rightward axis deviation, best appreciated in lead aVR (Figure 9). Many sodium channel blockers have additional effects on other cardiac receptors and ion channels.1 Although poisoning from tricyclic antidepressants (TCAs) is best studied, many other poisons cause life-threatening sodium channel blockade.2 Treatment recommendations for poisoning by other sodium channel blockers are often extrapolated from TCA studies. Management of life-threatening poisoning from local anesthetics, whose pharmacologic action is similar to class Ib antiarrhythmics, is discussed in the Toxicology: Local Anesthetics section. The treatment of cocaine poisoning, which has toxicity unique from other local anesthetics, is discussed in the Toxicology: Cocaine section. Management of chloroquine and hydroxychloroquine poisoning, which is unique, is outside the scope of these guidelines.
These recommendations inform the management of adults and children with life-threatening sodium channel blocker poisoning, including cardiac arrest.
Recommendation-Specific Supportive Text
1 and 2. Sodium loading and increasing the serum pH are each supported for the treatment of hypotension and arrhythmia from TCA poisoning by in vitro studies, animal studies, and case series and cohort studies of adults and children.3-6 Administration of hypertonic sodium bicarbonate achieves both of these physiologic goals, though its mechanism is not fully elucidated. For dosing information, refer to Table 4. This practice is supported by case series in TCA poisoning, involving mostly adults.4,5 Whether the target endpoints of initial hypertonic sodium bicarbonate therapy bolus therapy should be biochemical (pH ≈7.50) or physiologic (resolution of hypotension and QRS prolongation) is debated.3,7-9 After the goals of alkalinization are achieved, it is not known whether it is superior to monitor the patient and administer additional sodium bicarbonate boluses as needed or start a continuous infusion.6 Experts recommend avoiding extremes of hypernatremia (serum sodium not to exceed 155 mEq/L) and alkalemia (serum pH not to exceed 7.55) to avoid iatrogenic harm.3,6,10 If pH exceeds 7.55 and serum sodium is below 155 mEq/L, serum sodium can be increased by administration of hypertonic saline; if serum sodium exceeds 155 mEq/L and pH is below 7.55, pH can be controlled by adjusting minute ventilation in intubated adults and children.5,11,12
3. Observational data from adults and children (≥15 years of age) suggest that patients with tricyclic antidepressant poisoning who are treated with mechanical hyperventilation in addition to sodium bicarbonate may be more likely to achieve controlled alkalemia and correction of QRS prolongation compared with those receiving sodium bicarbonate alone.5 The clinical threshold for intubation is unclear; in adults and children who are intubated, minute ventilation can be adjusted to achieve pH targets.5,11
4. ECLS, including VA-ECMO, has been used successfully in adult cases with refractory cardiogenic shock from sodium channel blocker poisoning.13-17 One retrospective cohort study comparing 14 poisoned adults (7 poisoned by sodium channel blockers) treated with ECLS with 48 poisoned adults (14 poisoned by sodium channel blockers) with conventional therapies found that ECLS was associated with a lower mortality rate in the overall cohorts.18 The study did not have a subgroup analysis of only sodium channel blocker poisoning. There is a single case report of a child with cardiac arrest secondary to TCA poisoning who was successfully treated with VA-ECMO.19 No controlled observational studies or clinical trials were identified. Further discussion of the use of VA-ECMO in poisoning is provided in the Toxicology: ECMO section.
5. The use of sodium bicarbonate to treat sodium channel blocker poisoning other than from TCAs is based on case reports of adult poisoning by other sodium channel blockers.3,20-23 No pediatric data were identified, thus, the recommendation for children is extrapolated from the adult data. For dosing information in adults and children, refer to Table 4.
6. Lidocaine, a class Ib antidysrhythmic, competes with Ia and Ic antiarrhythmics for binding at the sodium channel. Class Ib antiarrhythmics dissociate from the receptor more rapidly than Ia or Ic agents, such as TCAs, and, therefore, do not depress phase 0 depolarization.24 The use of lidocaine to treat wide-complex tachycardia from TCA overdose is supported by animal studies and case reports of adults and children.24 A similar role for phenytoin, another class Ib antiarrhythmic, is supported by adult case reports, though not consistently by animal studies.24-27
7. Most sodium channel blockers are highly lipophilic. Several case reports in adults and children describe temporal improvement following ILE administration, including successful treatment of TCA-induced cardiac arrest.28-37 However, an RCT published in abstract form only found no benefit from ILE administration in treatment of hypotension or electrocardiogram abnormalities from TCA poisoning.38 The administration of ILE may increase drug absorption in oral overdose in an animal model, and results of animal studies are inconsistent.39-43 Nonetheless, existing evidence-based recommendations suggest ILE administration after failure of standard therapies for life-threatening amitriptyline poisoning, particularly if VA-ECMO or other forms of mechanical circulatory support are not readily available.44
The hallmark of sympathomimetic poisoning is increased activity of the adrenergic nervous system. Amphetamines, cathinones, and some synthetic cannabinoids produce sympathomimetic poisoning. Management of severe cocaine poisoning is discussed separately (refer to the Toxicology: Cocaine section). Complications of sympathomimetic poisoning result from excessive catecholamine release and an attendant increase in metabolic and psychomotor activity. Clinical manifestations include tachycardia, hypertension, agitation, seizures, hyperthermia, rhabdomyolysis, and acidosis.1-4
Sympathomimetic poisoning can cause sudden cardiac arrest, presenting as VF, VT, or PEA.5-9 Vasospasm can cause myocardial infarction, even in patients with normal coronary arteries.6,8,10,11 A stress (takotsubo) cardiomyopathy is also reported in sympathomimetic-poisoned patients; this condition can be fatal, but it resolves spontaneously in survivors.10,12-15 Hyperthermia is a severe and rapidly life-threatening clinical manifestation.3-5,16,17
These recommendations inform the management of adults and children with life-threatening sympathomimetic poisoning, including cardiac arrest.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Sedation is recommended for adults with severe agitation from sympathomimetic poisoning. |
| 1 | C-LD | 2. Rapid external cooling with ice water immersion is recommended for adults with life-threatening hyperthermia from sympathomimetic poisoning. |
| 2a | C-EO | 3. Vasodilators (eg, nitrates, phentolamine, CCBs) are reasonable for adults with coronary vasospasm from sympathomimetic poisoning. |
| 2a | C-EO | 4. It is reasonable to use ECLS in adults with cardiogenic shock from sympathomimetic poisoning refractory to other treatment measures. |
| 2b | C-LD | 5. The usefulness of dantrolene is uncertain for adults with life-threatening hyperthermia from MDMA toxicity. |
| 3: Harm | C-LD | 6. Prolonged use of physical restraint without sedation for adults is potentially harmful. |
Adults with Sympathomimetic Poisoning
Recommendation-Specific Supportive Text
- Sedatives (eg, benzodiazepines, antipsychotics) have been used in animal experiments to treat sympathomimetic poisoning.18,19 Sedatives control psychomotor agitation that produces heat and rhabdomyolysis. Antipsychotics control agitation. Benzodiazepines mitigate agitation, relax muscles, and treat seizures. Although several adult clinical trials compare specific therapies for severe psychomotor agitation, it is difficult to separate patients with sympathomimetic poisoning from other patients in these studies, and cardiac arrest was rare.20-24
- Hyperthermia is rapidly life-threatening in sympathomimetic poisoning.25 External and immersive cooling has been used to treat hyperthermia in adults and a 17-year-old patient with sympathomimetic poisoning.5,26-28 Evaporative or immersive cooling modalities reduce temperature more rapidly in adults than cooling blankets, the application of cold packs, or endovascular cooling devices.29-33 Ice water immersion resulted in the fastest cooling rates in adults and critically may cool faster than 0.15 oC/min (0.27 oF/min), which has been associated with increased survival.34,35 High-quality data on cooling measures for hyperthermic sympathomimetic patients are lacking, but immersion seems effective (refer to the Hyperthermia section).28
- Vasodilators, including alpha-adrenergic receptor antagonists, CCBs, and nitrates, have been used to treat coronary vasospasm, reversing electrocardiographic and biochemical markers of ischemia in sympathomimetic-poisoned adults.36-38
- ECLS, including VA-ECMO and intra-aortic balloon pump, has been used successfully to support cardiac output in adults in cardiogenic shock while stress cardiomyopathy resolves.10,13,39,40 Stress (takotsubo) cardiomyopathy can be fatal, but it often spontaneously resolves in days to weeks with circulatory support.
- Data on dantrolene for MDMA-induced hyperthermia is conflicting. A meta-analysis of patients aged ≥15 years only identified case reports and series totaling 71 cases and found that dantrolene use was associated with improved survival, but a nonrandomized series of 9 adults with MDMA-induced hyperthermia did not find clinical differences between patients who received dantrolene and those who did not.41,42
- Although physical restraints may be necessary temporarily, their sustained use without effective sedation is associated with death in adults with severe agitation from exposure to cocaine and other sympathomimetics.37,43-45
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Sedation is recommended for children with severe agitation from sympathomimetic poisoning. |
| 1 | C-EO | 2. Rapid external cooling with ice water immersion is recommended for children with life-threatening hyperthermia from sympathomimetic poisoning. |
| 2a | C-EO | 3. Vasodilators (eg, nitrates, phentolamine, CCBs) are reasonable for children with coronary vasospasm from sympathomimetic poisoning. |
| 2a | C-EO | 4. It is reasonable to use ECLS in children with cardiogenic shock from sympathomimetic poisoning refractory to other treatment measures. |
| 2b | C-EO | 5. The usefulness of dantrolene is uncertain for children with life-threatening hyperthermia from MDMA toxicity. |
| 3: Harm | C-EO | 6. Prolonged use of physical restraint without sedation for children is potentially harmful. |
Children with Sympathomimetic Poisoning
Recommendation-Specific Supportive Text
- Sedatives (eg, benzodiazepines, antipsychotics) have been used in animal experiments to treat sympathomimetic poisoning.18,19 Sedatives control psychomotor agitation that produces heat and rhabdomyolysis. Antipsychotics control agitation. Benzodiazepines mitigate agitation, relax muscles, and treat seizures. Although several adult clinical trials compare specific therapies for severe psychomotor agitation, it is difficult to separate patients with sympathomimetic poisoning from other patients in these studies, and cardiac arrest was rare.20-24 No high-quality pediatric data on the sedatives for sympathomimetic were identified, thus, the recommendation for children is extrapolated from the adult data.
- Hyperthermia is rapidly life-threatening in sympathomimetic poisoning.25 External and immersive cooling has been used to treat hyperthermia in adults and a 17-year-old patient with sympathomimetic poisoning.5,27,28 Evaporative or immersive cooling modalities reduce temperature more rapidly in adults than cooling blankets, the application of cold packs, or endovascular cooling devices.29-33 Ice water immersion resulted in the fastest cooling rates in adults and critically may cool faster than 0.15 °C/min (0.27 °F/min), which has been associated with increased survival.34,35 High-quality data on cooling measures for hyperthermic sympathomimetic patients are lacking, but immersion seems effective.28 Limited pediatric data on the rapid external cooling for life-threatening sympathomimetic poisoning were identified, thus, the recommendation for children is extrapolated primarily from the adult data (refer to the Hyperthermia section).
- Vasodilators, including alpha-adrenergic receptor antagonists, CCBs, and nitrates, have been used to treat coronary vasospasm, reversing electrocardiographic and biochemical markers of ischemia in sympathomimetic-poisoned adults.36-38 No pediatric data on the use of vasodilators for life-threatening sympathomimetic poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
- ECLS, including VA-ECMO and intra-aortic balloon pump, has been used successfully to support cardiac output in adults in cardiogenic shock while stress cardiomyopathy resolves.10,13,39,40 Stress (takotsubo) cardiomyopathy can be fatal, but it often spontaneously resolves in days to weeks with circulatory support. No pediatric data on the use of ECLS for life-threatening sympathomimetic poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
- Data on dantrolene for MDMA-induced hyperthermia is conflicting. A meta-analysis of patients aged ≥15 years only identified case reports and series totaling 71 cases and found that dantrolene use was associated with improved survival, but a nonrandomized series of 9 adults with MDMA-induced hyperthermia did not find clinical differences between patients who received dantrolene and those who did not.41,42 Limited pediatric data on the use of dantrolene to treat life-threatening sympathomimetic poisoning were identified, thus, the recommendation for children is extrapolated primarily from the adult data.
- Although physical restraints may be necessary temporarily, their sustained use without effective sedation is associated with death in adults with severe agitation from exposure to cocaine and other sympathomimetics.37,43-45 No pediatric data on the use of physical restraint for life-threatening sympathomimetic poisoning were identified, thus, the recommendation for children is extrapolated from the adult data.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-EO | 1. It may be reasonable to use β-adrenergic antagonists for adults and children with ventricular arrhythmias refractory to standard therapy. |
Synopsis
Cardiac arrest following volatile hydrocarbon use has been termed sudden sniffing death.1 While the exact mechanism is unknown, in vitro and animal experiments suggest that hydrocarbons sensitize cardiac potassium, sodium, and calcium channels.2-5 Hypoxia, catecholamine surge, or hypocalcemia (from fluorinated hydrocarbons) may also contribute to lethal arrhythmias. Animals treated with chloroform, a nonhydrocarbon volatile, have enhanced sensitivity to epinephrine, which led to death in some animals.6,7 In a large case series, sudden death was frequently reported following exertion or a stressful situation.1
This recommendation informs the management of adults and children with life-threatening arrhythmias from volatile hydrocarbon exposure, including cardiac arrest.
Recommendation-Specific Supportive Text
- β-adrenergic antagonists decrease myocardial sensitivity to epinephrine. Several case reports describe resuscitation with a β-adrenergic antagonist for cardiac arrest from volatile hydrocarbons. A 23-year-old patient suffered pulseless VT cardiac arrest following inhalation of 1,1-difluoroethane. In addition to amiodarone and defibrillation, recurrent VT was successfully treated with calcium, dexmedetomidine, esmolol, and cardiac bypass.8 Another adult case report described myocardial infarction and VT after inhalation of a keyboard cleaner containing difluoroethane. Despite amiodarone, lidocaine, and metoprolol, the patient died of refractory cardiogenic shock.9 The use of β-adrenergic antagonists is further supported by the extrapolation of data from withholding epinephrine. In a case series of 22 patients aged ≥13 years with cardiac arrest from volatile hydrocarbons, none of the 8 patients receiving epinephrine survived, while 5 of the 6 patients who did not receive epinephrine survived with good neurological outcome.10
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. It is reasonable to use VA-ECMO in adults and children with persistent cardiogenic shock or cardiac arrest due to poisoning that is refractory to pharmacological interventions. |
| 2a | C-EO | 2. It is reasonable to use VA-ECMO in adults and children with persistent arrhythmias due to poisoning when other treatments fail. |
| 2b | C-LD | 3. For adults and children with life-threatening poisoning, the effectiveness of VA-ECMO for poisoned patients with cardiovascular collapse from causes other than cardiogenic shock or arrhythmia has not been established. |
Synopsis
VA-ECMO is a resuscitative measure providing both cardiac and pulmonary support.1 In the setting of poisoning, VA-ECMO treats refractory cardiogenic shock by providing mechanical circulatory support while the offending poison is eliminated. The use of VA-ECMO in both poisoned adults and children is limited by availability, logistics of transport, patient comorbidities, and risks inherent to the procedure. Decisions to initiate VA-ECMO involve the understanding of both the pathophysiology of the specific poisoning and the clinical features of the patient and may include consultation from a poison center or medical toxicologist.
Other forms of mechanical circulatory support, such as implanted LVADs and percutaneous mechanical circulatory support devices (eg, intra-aortic balloon pump) are beyond the scope of this recommendation.
These recommendations inform the management of adults and children with life-threatening poisoning, including cardiac arrest.
Recommendation-Specific Supportive Text
- In a retrospective cohort study of 64 adults treated with VA-ECMO for cardiac arrest or refractory shock regardless of cause, cardiotoxic poisoning was independently associated with survival.2 A nonrandomized observational study of adults in cardiac arrest due to poisoning treated with VA-ECMO showed lower mortality than other patients treated with VA-ECMO and lower mortality compared with poisoned patients treated with standard critical care and antidotal therapy alone.3 In a prospective observational study of 32 adults placed on VA-ECMO to treat cardiogenic shock or refractory cardiac arrest from poisoning, 78% of patients survived to hospital discharge.4 A review of 407 patients with poisoning treated with either VA-ECMO or venovenous ECMO reported to the National Poison Data System© included 75 patients less than 12 years of age and found similar mortality rates for adults and children (29.5% versus 32.0%, respectively).5 Significant complications with VA-ECMO treatment include limb ischemia, bleeding, stroke, and infection.1,4,6 Studies have reported complications of VA-ECMO following administration of lipid emulsion therapy, including cracking of stopcocks, clogging and malfunction of the membrane oxygenator, and increased blood clot and lipid aggregation in the circuits.7,8 However, multiple case reports reported successful treatment with VA-ECMO concurrent with lipid emulsion therapy.8,9
- For adults and children with persistent nonperfusing arrhythmias, VA-ECMO provides antegrade blood flow to allow poison elimination. Case reports describe neurologically intact survival with the use of VA-ECMO to support adult cases of poisoning with persistent arrhythmias.10-13 One adult case resulted in return to normal sinus rhythm, but the patient subsequently suffered multisystem organ failure.14 Similar to adults, there are case series and case reports of the use of VA-ECMO to treat children with persistent arrhythmias following poisoning.15,16
- The mortality benefit of VA-ECMO is mixed in poisonings that cause refractory vasodilatory shock with preserved cardiac function, direct cellular toxicity, disruption of cellular oxygen use, or poisonings that are almost universally fatal despite temporary cardiac support. One retrospective registry study of patients reported to the National Poison Data System©, including 75 children less than 12 years of age, showed that patients with hematological and metabolic poisons had higher mortality on ECMO compared with patients with other poisonings; however, this study was unable to determine the mode of ECMO utilized.5 In contrast, in 1 retrospective case series, 80% of children less than 18 years of age who were treated with VA-ECMO (with or without continuous kidney replacement therapy) for vasodilatory shock from poisoning survived.17 Similarly, multiple case reports have demonstrated mortality benefit of VA-ECMO for adults with cardiogenic shock, hypotension, and arrhythmias in the setting of aluminum phosphide poisoning, a pesticide and rodenticide that when exposed to water produces phosphine gas, a direct cellular toxin that causes depression of myocardial contractility and ventricular arrhythmias.18 The pathophysiology of these poisons is not expected to differ significantly between adults and children, including aluminum phosphide.
Cardiac arrest and life-threatening states due to special circumstances are vastly under-researched. As part of the overall work for the development of these guidelines, the writing group reviewed a large amount of literature concerning the management of cardiac arrest and life-threatening states due to special circumstances. One expected challenge faced through this process was the lack of data in many areas. With some exceptions, cardiac arrests due to special circumstances are rare events and challenging to study. As a result, only the majority of guideline recommendations were based on low-grade evidence (LOE C).
Some critical knowledge gaps identified by the writing group are summarized in Table 5.
Table 5. 2025 Adult and Pediatric Special Circumstances of Resuscitation Key Knowledge Gaps
| Alternative CPR |
|---|
|
| Anaphylaxis |
|
| Asthma |
|
| Cardiac intervention laboratory |
|
| Cardiac surgery |
|
| Drowning |
|
| Electrocution |
|
| Gas embolism |
|
| High-consequence respiratory pathogens |
|
| Hyperkalemia |
|
| Hyperthermia |
|
| Hypothermia |
|
| Left ventricular assist device |
|
| Pregnancy |
|
| PE |
|
| Benzodiazepines |
|
| β-blockers and CCBs |
|
| Cocaine |
|
| Cyanide |
|
| Digoxin |
|
| Local anesthetics |
|
| Methemoglobinemia |
|
| Opioids |
|
| Organophosphates and carbamates |
|
| Sodium channel blockers |
|
| Sympathomimetics |
|
| Volatiles |
|
| Role of VA-ECMO in poisoning |
|
AED indicates automated external defibrillator; ALS, advanced life support; β-blockers, β-adrenergic receptor antagonists; CCBs, calcium channel blockers; CPR, cardiopulmonary resuscitation; Fab, fragment antigen binding; ECLS, extracorporeal life support; ECPR, extracorporeal cardiopulmonary resuscitation; ILE, intravenous lipid emulsion; LVAD, left ventricular assist device; PE, pulmonary embolism; PPE, personal protective equipment; RCT, randomized controlled trial; TCA, tricyclic antidepressant; VA-ECMO, venoarterial extracorporeal membrane oxygenation; and VF, ventricular fibrillation.
The American Heart Association requests that this document be cited as follows: Cao D, Arens AM, Chow SL, Easter SR, Hoffman RS, Lagina AT III, Lavonas EJ, Patil KD, Sutherland LD, Tijssen JA, Wang GS, Zelop CM, Rodriguez AJ, Drennan IR, McBride ME. Part 10: adult and pediatric special circumstances of resuscitation: 2025 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2025;152(suppl2):S578–S672. doi: 10.1161/CIR.0000000000001380
- Dazhe Cao, MD, Chair
- Ann M. Arens, MD
- Sheryl L. Chow, PharmD
- Sarah Rae Easter, MD
- Robert S. Hoffman, MD
- Anthony T. Lagina, MD
- Eric J. Lavonas, MD, MS
- Kaustubha D. Patil, MD
- Lauren D. Sutherland, MD
- Janice A. Tijssen, MD, MSc
- George Sam Wang, MD
- Carolyn M. Zelop, MD
- Amber J. Rodriguez, PhD
- Ian R. Drennan, ACP, PhD
- Mary E. McBride, MD, MEd, Vice Chair
Contributors:
Amber V. Hoover, RN, MSN; Andrea Nillas, MD; Philip Jarrett, MD, MBA; Avi Ruderman, MD; Matthew D. McEvoy, MD; Michael R. Fettiplace, MD, PhD; Mark S. Link, MD; Matthew R. Neth, MD; Gurpreet S. Dhillon, MD; Marina Del Rios, MD, MS; Jason A. Bartos, MD, PhD; Ashish R. Panchal, MD, PhD; Comilla Sasson, MD, PhD
We acknowledge the contributions of Nhat Chau, MD; Bhargavesh Gottam, MD; and Olivia Williams, MD, who assisted the authors in the evidence review of these recommendations.
We acknowledge the contributions of the librarians Abdul Pullattayil, Amy Sisson, Caitlin Plovnick, Joey Nicholson, Laura Haygood, and Peace Ossom, who assisted with systematic searches.
Open table in a new window.
Open table in a new window.
 Login
Login