Part 7: Adult Basic Life Support
Abstract
The American Heart Association’s 2025 Adult Basic Life Support Guidelines build upon prior versions with updated recommendations for assessment and management of persons with cardiac arrest, as well as respiratory arrest and foreign-body airway obstruction. The chapter addresses the important elements of adult basic life support including initial recognition of cardiac arrest, activation of emergency response, provision of high-quality cardiopulmonary resuscitation, and use of an automated external defibrillator. In addition, there are updated recommendations on the treatment of foreign-body airway obstruction. The use of opioid antagonists (eg, naloxone) during respiratory or cardiac arrest is incorporated into the adult basic life support algorithms, with more detailed information provided in “Part 10: Adult and Pediatric Special Circumstances of Resuscitation.”
Top 10 Take-Home Messages
- In adult cardiac arrest, resuscitation should generally be conducted where the patient is found, as long as high-quality cardiopulmonary resuscitation (CPR) can be administered safely and effectively.
- After identifying an adult in cardiac arrest, a lone responder should activate the emergency response system first, then immediately begin CPR.
- In adult cardiac arrest, rescuers should perform chest compressions with the patient’s torso at approximately the level of the rescuer’s knees.
- It is reasonable for health care professionals to perform chest compressions and ventilations for all adult patients in cardiac arrest from either a cardiac or noncardiac cause.
- When ventilating adult patients in cardiac arrest, it is reasonable to give enough tidal volume to produce visible chest rise while avoiding hypo- and hyperventilation.
- The routine use of mechanical CPR devices is not recommended for adults in cardiac arrest.
- For adult patients who are not breathing normally but have a pulse, it is reasonable for rescuers to provide 1 breath every 6 seconds (10 breaths per minute).
- CPR for adult cardiac arrest patients with obesity should be provided by using the same techniques as for the average weight patient.
- For adults with severe foreign-body airway obstruction (FBAO), rescuers should perform cycles of 5 back blows followed by 5 abdominal thrusts until the object is expelled or the patient becomes unresponsive.
- During adult cardiac arrest, it is reasonable for rescuers to use personal protective equipment (PPE) while performing CPR.
Preamble
The annual incidence of adults treated by emergency medical services (EMS) for out-of-hospital cardiac arrest (OHCA) in the United States varies considerably between states, but is estimated at 356 000, or 83 per 100 000 populations.1,2 Despite advances in public education and awareness, as well as improvement in community-based systems of care, survival for adults after OHCA remains low and decreased during the COVID-19 pandemic.3 The Cardiac Arrest Registry to Enhance Survival (CARES) is a voluntary OHCA database used by EMS agencies and hospitals to generate Utstein-style reports and to benchmark performance and outcomes against similar systems. Developed in 2004, CARES now has participating sites from 37 states covering approximately 56% of the US population. CARES OHCA data from 2024 showed that survival to hospital discharge was 10.5%, with favorable neurologic outcome reported in approximately 8.2%.4 The majority of adult OHCA occurred in private residences while 18% occurred in public places. Bystander CPR was provided in 47.7% of adult OHCA, and a bystander used an automated external defibrillator (AED) in 7.9% of cases. There is significant variation in rates of bystander CPR, public AED use, EMS response times, and survival from cardiac arrest between geographic regions, as well as disparities associated with race, sex, and socioeconomic status.5,6
The annual incidence of adult in-hospital cardiac arrest (IHCA) in the United States is estimated to be 292 000 by extrapolation from the Get With The Guidelines-Resuscitation registry.7 Approximately 60% of adult IHCA occur in an acute care setting (eg, intensive care unit, emergency department, operating room) while 40% occur on the general inpatient units. Survival to hospital discharge decreased from 26.7% to a low of 18.8% during the COVID-19 pandemic, with improvement to 23.6% in 2023.1 Racial and sex-related outcome disparities have also been observed in the IHCA setting.8,9
Early, high-quality CPR and prompt defibrillation are the most important interventions associated with improved outcomes in adult cardiac arrest. Despite this, a 2015 US prevalence report found that only 18% of people surveyed had current CPR training,10 with lower rates in under-represented and low-income populations. More lives could be saved if a greater proportion of the public was trained in, and willing to perform, basic life support, especially chest compressions.11
Since 2010, the American Heart Association (AHA) Emergency Cardiovascular Care (ECC) Committee has regularly set goals aimed at increasing survival from cardiac arrest. The accompanying strategies focus on strengthening the links in the Chain of Survival to prevent, identify, treat, and support all phases of care for persons who are at risk for, or experience, cardiac arrest. The fundamental basic life support tasks of recognition of cardiac arrest, activation of emergency response, performance of chest compressions and ventilations, and use of an AED for defibrillation are critical components representing the first links of the Chain of Survival that must be optimized so persons with cardiac arrest can fully benefit from advanced cardiovascular care therapies.12
The 2030 Impact Goals focus on improving survival to hospital discharge with favorable neurologic outcome for individuals experiencing OHCA or IHCA.13 Not surprisingly, the first 2 goals are related to basic life support: bystander CPR and public access defibrillation. Specifically, the first goal calls for an increase in bystander adult CPR performance rates to greater than 50%, while the second goal is to increase the proportion of adults with cardiac arrest for whom an AED is applied before emergency medical response arrival to greater than 20%. To accomplish these goals, the evidence-based recommendations for performance of high-quality basic life support provided in this chapter must be coupled with strategies for awareness, advocacy, and education that improve the system of care for all persons. The accompanying chapters “Part 12: Resuscitation Education Science” and “Part 4: Systems of Care” provide recommendations for optimizing the community and health care system approach to cardiac arrest treatment, including bystander CPR training, telecommunicator CPR, public access defibrillation, and timely activation of the emergency medical response system. While all components of the Chain of Survival are essential, high-quality basic life support is foundational to improving outcomes.
Introduction
These recommendations supersede the last full set of AHA Guidelines for Adult Basic Life Support published in 202014 unless otherwise specified. The writing group reviewed all relevant and current AHA Guidelines for CPR and ECC and all relevant International Liaison Committee on Resuscitation (ILCOR) consensus on CPR and ECC science with treatment recommendations from 2020 through 2024.15-18 Evidence and recommendations were reviewed to determine if current guidelines should be reaffirmed, revised, or retired, or if new recommendations were needed. The writing group then drafted, reviewed, and approved each recommendation. For topics that did not undergo full evidence review or updated literature search, the recommendations, recommendation-specific supportive text, and references from the 2020 Basic Life Support Guidelines were not updated and were carried over. These topics are noted within the synopsis of their respective sections and remain as the current guidelines for 2025.
Scope of the Guidelines
The 2025 Adult Basic Life Support Guidelines apply to a range of responders, including trained and untrained lay rescuers and health care professionals, with the understanding that systems of prehospital and in-hospital care vary widely across the world. They address the treatment of cardiac arrest as well as other immediately life-threatening conditions including respiratory arrest and FBAO. A person in cardiac arrest who has signs of puberty is treated by using the Adult Basic Life Support Guidelines; guidelines for pediatric patients are discussed in “Part 6: Pediatric Basic Life Support.”
Organization of the Adult Basic Life Support Writing Group
The Adult Basic Life Support Writing Group was composed of subject matter experts and investigators with diverse backgrounds in prehospital care, emergency medicine, critical care medicine, and respiratory care in both urban and rural North American settings. Before selection for the writing group, members disclosed any conflicts of interest, either financial or intellectual (see Appendix), and a robust conflict of interest process was followed (see “Part 2: Evidence Evaluation and Guidelines Development”).
Methodology and Evidence Review
The AHA is an active member of ILCOR, a consortium of 8 member councils that collaborates to review resuscitation literature and develop a consensus on science. When possible, ILCOR also provides treatment recommendations for specific aspects of ECC.
The Adult Basic Life Support Guidelines are based on the rigorous evidence evaluation process undertaken by ILCOR which includes evidence updates, scoping reviews, and systematic reviews. When a specific topic is not concurrently addressed by ILCOR, the writing group performed updated evidence evaluations by using standardized databases to capture any additional relevant studies since the last review. More details are provided in “Part 2: Evidence Evaluation and Guidelines Development.”
Class of Recommendation and Level of Evidence
As in the past, all 2025 AHA Adult Basic Life Support Guideline recommendations are classified according to the AHA criteria for Class of Recommendation (COR) and Level of Evidence (LOE; Table 1). The COR refers to the strength and consistency of the evidence reflects the writing group’s synthesis of the available literature, the potential impact on patient outcome, and the relevant principles of feasibility, acceptability, and equity. The specific wording is intended to reflect the risk-benefit ratio associated with the recommendation, with terms such as “is recommended” or “should” reserved for Class 1 recommendations.
The LOE reflects the quality of the evidence, with highest value (LOE A) placed on randomized controlled trials (RCTs) and meta-analyses involving RCTs. Lower-quality randomized, nonrandomized, or registry studies, and observational studies are categorized as lower levels of evidence. Recommendations based on limited data or expert opinion are indicated as LOE C.
The use of COR and LOE for each recommendation provides transparency to the guideline user to distinguish between those recommendations that are considered clearly effective and those for which more high-quality research may result in future guideline revisions. By acknowledging the limitations of the available evidence, the guidelines are intentionally identifying knowledge gaps and areas of uncertainty to be addressed by further investigation.
Open table in a new window.
Guideline Structure
The 2025 Adult Basic Life Support Guidelines are organized into knowledge chunks, which are tables of recommendations covering specific topics. Each individual recommendation has a color-coded corresponding COR and LOE to indicate the strength of the recommendation and the type and quality of evidence supporting it. A brief synopsis follows the table to provide context and key concepts. The recommendation-specific supporting text provides background information on the topic as well as specific key studies or trials that were considered.
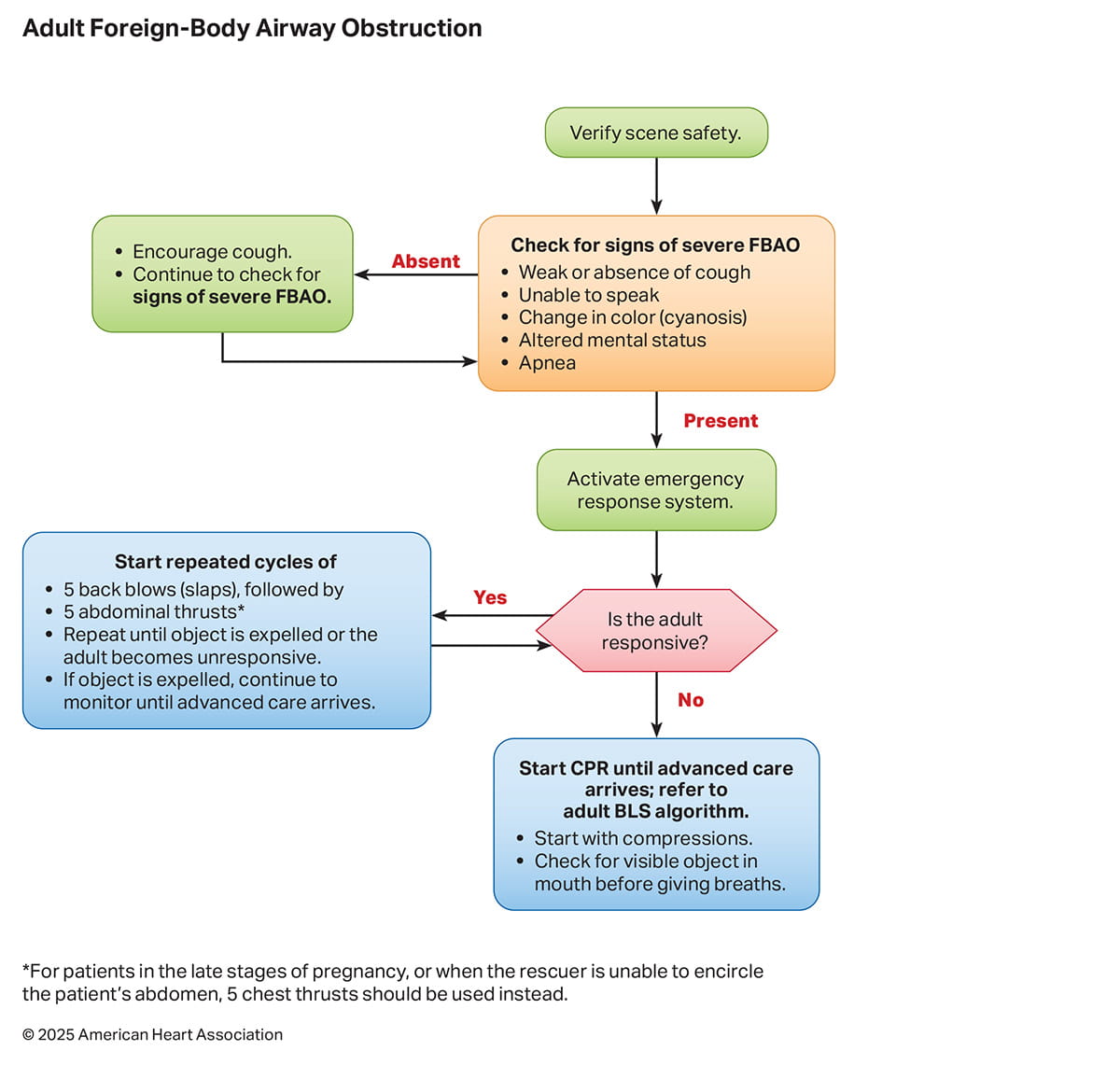
Of note, the order of recommendations in the tables is determined primarily by the COR, with Class 1 recommendations first, then by LOE category. The order is not intended to reflect the sequence of resuscitation step performance, which is illustrated in the accompanying algorithms: Adult Basic Life Support for Health Care Professionals (Figure 1), Adult Basic Life Support for Lay Rescuers (Figure 2), and Adult Foreign-Body Airway Obstruction (Figure 3).
Document Review and Approval
Each Adult Basic Life Support Writing Group member reviewed and approved each recommendation provided in the 2025 Adult Basic Life Support Guidelines. In addition to the writing group, the guidelines were reviewed by the ECC Science Oversight Committee and were submitted for blinded peer review by subject matter experts. All participants in the writing and review process were required to disclose conflicts of interest including relationships with industry. Additional review by the AHA Scientific Advisory and Coordinating Committee and the AHA Executive Committee occurred before journal submission. Disclosures for writing group members and reviewers are provided in Appendixes 1 and 2.
Updated AHA Algorithms for Adult Basic Life Support and Foreign-Body Airway Obstruction
Three algorithms are included in the 2025 Guidelines as resources. The Adult Basic Life Support for Health Care Professionals Algorithm (Figure 1) now incorporates the use of opioid antagonists for both respiratory and cardiac arrest. A new adult basic life support algorithm (Figure 2) illustrates the approach for lay rescuers. A new algorithm for assessment and treatment of FBAO (Figure 3) is also provided.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. If an adult is unconscious/unresponsive, with absent or abnormal breathing (ie, only gasping), the lay rescuer should assume the person is in cardiac arrest. |
| 1 | C-LD | 2. If an adult is unconscious/unresponsive, with absent or abnormal breathing (ie, only gasping), the health care professional should check for a pulse for no more than 10s and, if no definite pulse is felt, should assume the person is in cardiac arrest. |
Synopsis
Recognition of cardiac arrest can be difficult, especially in the out-of-hospital setting.1 Accurate detection of a pulse is challenging for all levels of responders, increasing the risk for delays in initiation of chest compressions and activation of emergency medical response. Recognition by lay rescuers is, therefore, based primarily on level of consciousness and respiratory effort rather than using a pulse check. Health care professionals are encouraged to check for a pulse as one component of the recognition of cardiac arrest; however, the emphasis is on prompt initiation of CPR if a pulse is not definitively felt.
Recommendation-Specific Supportive Text
- Assessment of patient unresponsiveness and absent or abnormal breathing have been shown to rapidly identify a significant proportion of patients who are in cardiac arrest.2 Agonal breathing is characterized by slow, irregular gasping respirations that are ineffective for ventilation. Agonal breathing is described by lay rescuers with a variety of terms including abnormal breathing, snoring respirations, and gasping.3 Agonal breathing is common, reported as being present in up to 40% to 60% of OHCA, and diminishes the longer a person is in cardiac arrest.4,5 The presence of agonal breathing is cited as a common reason for lay rescuers to misdiagnose a patient as not being in cardiac arrest,6 and may lead to delays in initiation of chest compressions. Furthermore, the risk of harm associated with providing chest compressions to an unconscious patient who is not in cardiac arrest is low.7-11 The benefit of providing CPR for someone in cardiac arrest far outweighs any risk associated with providing chest compressions to someone who is not.
- Protracted delays in CPR can occur when checking for a pulse at the outset of resuscitation efforts as well as between successive cycles of CPR. Health care professionals often take too long to check for a pulse and have difficulty determining if a pulse is present or absent.12-14 There is no evidence, however, that checking for breathing, coughing, or movement is superior to a pulse check for detection of circulation.15 Thus, health care professionals are directed to quickly check for a pulse and to promptly start compressions when a pulse is not definitively palpated within 10 seconds.
Lay Rescuer (Untrained or Trained)
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 11. All lay rescuers should provide chest compressions for adults with presumed cardiac arrest. |
| 1 | B-NR | 2. After identifying an adult in cardiac arrest, a lone responder should activate the emergency response system first, then immediately begin CPR, beginning with chest compressions. |
| 1 | C-LD | 3. For adults in cardiac arrest, a lone rescuer with a mobile phone should activate the emergency response system and immediately begin CPR, beginning with chest compressions |
| 2a | B-R | 4. For lay rescuers trained in performing CPR, it is reasonable to provide ventilation (breaths) in addition to chest compressions for an adult in cardiac arrest. |
| 2a | C-LD | 5.When immediately available, it is reasonable for rescuers to use PPE during CPR for adult cardiac arrest. |
Synopsis
The first link in the Chain of Survival for cardiac arrest includes prompt activation of the emergency response system. Given that most lay rescuers will likely have mobile phones with hands-free options, it is possible for lay rescuers to provide CPR and activate the emergency response system at nearly the same time. Alternatively, a second lay rescuer can be instructed to call 911. Activation of the emergency response system allows for provision of telecommunicator CPR, possible notification of other lay rescuers via crowd-sourced applications, and dispatch of the designated EMS agency.
Immediate chest compressions are critical to improve patient outcomes from OHCA, and a chest compression–only approach is appropriate if lay rescuers are untrained or unwilling to provide breaths. Because CPR with breaths may lead to improved outcomes for adults in comparison with chest compression–only CPR, trained rescuers are encouraged to provide breaths along with chest compressions. PPE provides an important barrier against certain infectious diseases, but lay responders may have limited access to PPE.
Recommendation-Specific Supportive Text
- Immediate initiation of chest compressions is one of the most impactful interventions for survival from cardiac arrest.1-3 In Japan, nationwide dissemination of chest compression–only CPR for lay rescuers was associated with an increase in the incidence of survival with favorable neurological outcome after OHCAs, likely due to an increase in lay rescuers providing CPR.4 Providing manual chest compressions for an unconscious patient not in cardiac arrest has not been associated with serious harm, as demonstrated in several observational studies.5-10 The risk-to-benefit ratio remains heavily in favor of initiating CPR for presumed cardiac arrest when compared to the significant harm of withholding CPR when a patient is in cardiac arrest.
- A previous large observational study (N=17 461)) found no difference in survival to hospital discharge between patients receiving CPR before a call and patients receiving CPR after a call to the emergency response system.7,11 Our recommendation values the practical considerations of timely emergency medical response dispatch and the availability and value of remote assistance to improve the quality of CPR.
- Use of the “hands-free” speaker feature on most cell phones, when and where available, can help with near-simultaneous activation of emergency response and initiation of CPR. In situations where a phone is not immediately available, local circumstances will determine decisions about delaying CPR to activate the emergency medical response system; however, the importance of timely CPR must be emphasized.
- Numerous observational studies and 1 large secondary analysis of an RCT found improved outcomes in patients with cardiac arrest who received both chest compressions and ventilations compared with those who received chest compressions only.4,12-14 Other observational studies have reported no difference in outcome for patients receiving compressions and ventilations compared with compression-only CPR.13,15-21 Given the potential benefit of including both compressions and ventilations during CPR, if lay rescuers are appropriately trained, they should be encouraged to deliver breaths with compressions.
- The impact of PPE on CPR performance is an important consideration for responders. Although transmission of disease during CPR is uncommon, the COVID-19 pandemic heightened awareness of the importance of protection of rescuers, especially from airborne pathogens such as respiratory viruses. Because CPR is considered an aerosol-generating procedure, the use of PPE has become the norm. Safety and protection of the rescuer are of utmost importance in responding to cardiac arrest. Rescuers must be aware, however, that the process of donning PPE may delay the initiation of CPR, and use of PPE has the potential to adversely affect CPR performance and increase rescuer fatigue.22 A 2023 systematic review and meta-analysis found no difference in survival (1 clinical study)23 nor any change in CPR performance (17 manikin studies), with donning of PPE in simulated cardiac arrest.24 Two pooled studies from the same meta-analysis showed worse fatigue scores when rescuers performed CPR while wearing PPE.25,26 More information is provided in “Part 10: Adult and Pediatric Special Circumstances of Resuscitation.”
Initiation of Resuscitation: Health Care Professional
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. During adult cardiac arrest, a lone health care professional should commence with chest compressions rather than with ventilation. |
| 2a | C-LD | 2. It is reasonable for health care professionals to perform chest compressions and ventilation for all adult patients in cardiac arrest from either a cardiac or noncardiac cause. |
Synopsis
The 2010 Guidelines for CPR and ECC included a major change for trained rescuers, who were instructed to begin the CPR sequence with chest compressions rather than with breaths (circulation, airway, and breathing versus airway, breathing, and circulation) to minimize the time to initiation of chest compressions.27 As health care professionals are trained to deliver compressions and ventilation, they are in a position to provide both during adult basic life support.
Recommendation-Specific Supportive Text
- The circulation, airway, and breathing approach for adults is supported by a 2024 ILCOR systematic review.1-3,7,28 Once chest compressions have been started, a single trained rescuer delivers breaths by mouth-to-mask or by bag-mask device to provide oxygenation and ventilation. Manikin studies demonstrate that starting with chest compressions rather than with ventilation is associated with faster times to chest compressions, breaths, and completion of the first CPR cycle.2,3,29
- Numerous studies have shown improved outcomes when ventilations are provided in addition to chest compressions for adults in cardiac arrest.4,12-14 Delivery of chest compressions without assisted ventilation for prolonged periods could be less effective than conventional CPR (compressions plus ventilation) because arterial oxygen content decreases as CPR duration increases. This concern is especially pertinent in the setting of asphyxial cardiac arrest.13 Health care professionals, with their training and understanding, can realistically tailor the sequence of subsequent rescue actions to the most likely cause of arrest.
Opening the Airway in Adults in Cardiac Arrest
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. For unresponsive adult patients, health care professionals and trained lay rescuers should open the airway by using a head tilt–chin lift maneuver when no cervical spine injury is suspected. |
| 2a | C-LD | 2. When managing the airway of an adult with known or suspected basal skull fracture or severe coagulopathy, an oropharyngeal airway is preferred compared with a nasopharyngeal airway. |
| 2b | C-EO | 3. The use of an airway adjunct (eg, oropharyngeal or nasopharyngeal airway) may be reasonable in unconscious (unresponsive) adult patients with no cough or gag reflex to facilitate delivery of ventilation. |
| 3: No Benefit | C-LD | 4. The routine use of cricoid pressure in adult cardiac arrest is not recommended. |
Synopsis
Opening the airway is a key component of basic life support for patients who are unresponsive with or without respiratory or cardiac arrest. Unresponsive individuals are at risk for airway obstruction primarily due to the tongue falling to the back of the oropharynx as the oropharyngeal muscles lose tone. Untreated airway obstruction can lead to hypoxia and hypercarbia, which may precipitate cardiac arrest. Alternatively, uncorrected airway obstruction may hinder resuscitation efforts. Airway adjuncts such as oropharyngeal and nasopharyngeal airways can improve airway patency by creating a passage between the tongue and the pharynx. However, these devices have contraindications with suspected facial trauma (nasopharyngeal airway) and an intact gag reflex (oropharyngeal airway). Rescuers need to consider the possibility of cervical spine injury when there is known or suspected trauma. Cricoid pressure has not been shown to have benefit and has the potential to interfere with air entry into the trachea during bag-mask ventilation.
Recommendation-Specific Supportive Text
- The head tilt–chin lift is an effective technique to open an airway as demonstrated in noncardiac arrest and radiological studies (Figure 4).1-4 No studies have compared head tilt–chin lift with other airway maneuvers to establish an airway during cardiac arrest.
- The benefit of an oropharyngeal airway compared with a nasopharyngeal airway in the presence of a known or suspected basilar skull fracture or severe coagulopathy has not been assessed in clinical trials. However, use of an oropharyngeal airway reduces the risk of intracranial passage that may occur with nasopharyngeal airway insertion. Two case reports observed intracranial placement of nasopharyngeal airways in patients with basilar skull fractures.5,6
- Oropharyngeal and nasopharyngeal airway adjuncts can be used to maintain a patent airway and facilitate appropriate ventilation by preventing the tongue from occluding the airway. Incorrect placement, however, can cause an airway obstruction by displacing the tongue to the back of the oropharynx.1-4,7,8 One retrospective study reported improved neurologic outcomes during IHCA with use of airway adjuncts (eg, oropharyngeal or nasopharyngeal airway) in combination with bag-mask ventilation.9
- There is no evidence that cricoid pressure facilitates ventilation or reduces the risk of aspiration in cardiac arrest patients.10 There is some evidence that in non–cardiac arrest patients, cricoid pressure may protect against aspiration and gastric insufflation during bag-mask ventilation.11-14 However, additional studies, including a recent systematic review, found that cricoid pressure failed to reduce aspiration and may impede the placement of an advanced airway.15-19
Opening the Airway After Head and Neck Trauma
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In cases of head and neck trauma, trained rescuers should open the airway of an adult by using a jaw thrust without head extension. |
| 1 | C-EO | 2. For an adult with head and neck trauma, if the airway cannot be opened with a jaw thrust and airway adjunct insertion, trained rescuers should open the airway by using a head tilt–chin lift. |
| 3: Harm | C-LD | 3. In the setting of an adult with head and neck trauma, lay rescuers should not use rigid cervical devices for spinal motion restriction because their use by untrained rescuers may be harmful. |
Synopsis
An adult with signs of head or neck trauma may have a cervical spine injury. Trained rescuers should attempt to open the airway using the jaw thrust technique because this maneuver produces less movement of the head and neck than a head tilt–chin lift. If it is not possible to achieve an open airway with a jaw thrust and insertion of an airway adjunct, trained rescuers should open the airway by using the head tilt–chin lift given the critical importance of oxygenation and ventilation. No new evidence was identified on this topic during the 2025 evidence review.
Recommendation-Specific Supportive Text
- If an unresponsive person has head or neck trauma, there is a possibility of cervical spinal injury. Trained rescuers should open the airway by using a jaw thrust instead of head tilt–chin lift.1
- Maintaining a patent airway and providing adequate ventilation and oxygenation are priorities during CPR. If a jaw thrust and insertion of an airway adjunct are ineffective, a head tilt–chin lift may be necessary to open the airway. The importance of a patent airway outweighs the risk of further spinal damage in the cardiac arrest patient even in the setting of head and neck trauma.
- Manual spinal motion restriction can decrease movement of the cervical spine during patient care while allowing for proper ventilation and airway control.20,21 When head and neck injury are present, lay rescuers should maintain manual spinal motion restriction and should not use rigid cervical collar devices. Spinal immobilization devices such as rigid cervical collars may make it more difficult to maintain airway patency22,23 and provide adequate ventilation.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In adult cardiac arrest, rescuers should perform chest compressions with the patient’s torso at approximately the level of the rescuer’s knees whenever possible. |
| 1 | C-LD | 2. When providing chest compressions for an adult, the rescuer should place the heel of one hand on the center (middle) of the person’s chest (the lower half to lower third of the sternum) and the heel of the other hand on top of the first so that the hands are overlapped. |
| 1 | C-EO | 3. In adult cardiac arrest, resuscitation should generally be conducted where the person is found, as long as high-quality CPR can be administered safely and effectively. |
| 2a | C-LD | 4. In adult cardiac arrest, it is preferred to perform CPR on a firm surface and with the person in the supine position, when feasible and does not delay chest compressions. |
| 2b | C-LD | 5. In adult cardiac arrest, rescuers may consider placing their dominant hand on the sternum when performing chest compressions. |
| 2b | C-LD | 6. For adult patients who experience cardiac arrest while in the prone position, it may be reasonable to turn the patient supine before initiating chest compressions. If the patient cannot be safely turned supine, rescuers may consider performing CPR in the prone position. |
Synopsis
High-quality resuscitation is vital for improved cardiac arrest outcomes including return of spontaneous circulation (ROSC) and survival. The effectiveness of chest compression delivery can be improved through optimizing rescuer hand position, rescuer body position, and patient position (Figures 5 and 6).
Recommendation-Specific Supportive Text
- Rescuer position in relationship to the patient can affect chest compression quality. Multiple simulated cross-over RCTs have shown that kneeling on the floor or in the bed next to the manikin resulted in improved chest compression depth compared to standing in adult populations.1-6 Straddling the patient to perform CPR is similar to kneeling with improved chest compression quality in comparison to standing or walking alongside the patient while performing CPR on a moving cot7,8,9 However, kneeling and straddling, especially on a moving cot, needs to be weighed against rescuer safety and stability. One moderate-sized (n=124) RCT did not find benefit with straddling CPR compared to conventional lateral CPR while performed in a confined space.10 Two studies specifically evaluating height found that when the manikin torso was no more than 10 cm below the rescuer’s knee this was associated with improved chest compression depth.11,12 Using a step stool for rescuer elevation has also shown to improve chest compression quality in comparison to standing.1,4 Two RCTs demonstrated slightly higher chest compression quality metrics including depth and recoil with kneeling compared to step stool use.1,4
- A 2020 ILCOR systematic review identified 3 studies involving 57 patients that investigated the effect of hand positioning on resuscitation process and outcomes.13 No new studies were identified in a recent update of this review. Although no differences in resuscitation outcomes were noted, 2 studies found better physiological parameters (peak arterial pressure, mean arterial pressure, and end-tidal carbon dioxide) when compressions were performed over the lower third of the sternum compared with the middle of the sternum.14,15 A third study found no difference.16 Radiographic studies show the left ventricle is typically located inferior to the internipple line, corresponding with the lower half of the sternum.17 However, hand placement inferior to the internipple line may result in compression over the xiphoid, which may be less effective.18
- The ability to safely provide high-quality CPR should remain the highest priority when considering if a patient needs to be moved before initiation of CPR. If adequate chest compressions can be effectively provided while maintaining rescuer safety in the location the patient is found, immediate resuscitation can occur at this location. Importantly, delay in initiation of chest compressions is associated with worse outcomes. In a study of telephone-assisted CPR during OHCA, delays to CPR due to patient repositioning occurred in 41% of cases, most commonly due to physical limitations of the rescuer. Odds of survival to hospital discharge was significantly lower in the group with delayed chest compressions.19 This recommendation is not intended to apply to decision-making for the timing of transportation from the scene when a patient remains in cardiac arrest.
- A firm surface improves the likelihood of adequate chest compression depth. A recent systematic review including manikin studies recommended the use of a backboard in hospital settings to provide a firm surface for CPR.20 Similarly, compressions on a deflated dynamic mattress or use of bed manufacturer “CPR mode” in-hospital can improve chest compression depth.21,22 Studies have supported that optimal chest compressions are best delivered with the person on a firm surface such as the floor.23,24
- Dominant hand placement on the sternum may provide improved chest compression quality. One moderate-sized (n=101) RCT and 1 (n=225) observational study showed improved chest compression quality with dominant hand placement against the sternum.25-27 Another smaller RCT (n=59) found a non-significant improvement with dominant hand placement on the sternum.28
- While it is preferred to perform chest compressions with the patient in the supine position, there are circumstances in which the patient is in the prone position at the time of cardiac arrest. There are multiple case reports of performing resuscitation in the prone position for patients who experience cardiac arrest during operative procedures or while in the prone position for treatment of COVID-19–associated respiratory failure.29-31 A 2021 systematic review by ILCOR reported 20 adult patients with cardiac arrest in the prone position in the operating room or intensive care unit. Of the 12 patients who received CPR in the prone position, all experienced ROSC and survival to discharge. Of the 8 patients who were supinated before initiation of CPR, the rate of ROSC and survival to discharge were 37.5% and 29%, respectively.32
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In adult cardiac arrest, preshock and postshock pauses in chest compressions should be as short as possible. |
| 1 | C-LD | 2. During rhythm checks for adult cardiac arrest, the health care professional should minimize the time taken to check for a pulse (no more than 10 s), and if the rescuer does not definitely feel a pulse, chest compressions should be restarted immediately. |
| 2a | B-R | 3. In adults with cardiac arrest, it is reasonable to immediately resume chest compressions after shock administration rather than pause CPR to perform a postshock rhythm check. |
| 2a | B-R | 4. When 2 or more rescuers are available during an adult cardiac arrest, it is reasonable to switch chest compressors approximately every 2 min (or after about 5 cycles of compressions and ventilation at a ratio of 30:2). |
| 2a | C-LD | 5. For adults in cardiac arrest receiving CPR without an advanced airway, it is reasonable to pause compressions to deliver 2 breaths, each given over 1 s. |
| 2b | C-LD | 6. During adult cardiac arrest, it may be reasonable to perform CPR with a chest compression fraction of at least 60%. |
| 2b | C-LD | 7. Outside of the advanced life support environment, where invasive monitoring is available, there are insufficient data about the value of a pulse check while performing CPR for adult cardiac arrest. |
Synopsis
High-quality CPR is one of the most important interventions to improve survival from cardiac arrest. One key feature of high-quality CPR is to minimize interruptions in chest compressions, in addition to providing chest compressions of adequate rate and depth, allowing full chest recoil between compressions, and avoiding excessive ventilations.33,34 Chest compressions are required for forward flow during cardiac arrest and pauses in chest compressions have been shown to result in an almost immediate drop in coronary perfusion pressure, which is associated with reduced likelihood of ROSC.35,36 While some pauses in chest compressions, such as for ventilations and pulse checks, are necessary, ensuring that the number and duration of pauses are kept to a minimum is critical. The chest compression fraction (CCF) refers to the proportion of time a patient is receiving chest compressions during a resuscitation.
Recommendation-Specific Supportive Text
- An increase in the overall hands-on time (CCF) during resuscitation is directly associated with improved survival from cardiac arrest.37-39 Observational studies suggest improved outcomes with increased CCF in patients with shockable rhythms,40,41 as well as an association between ROSC and shorter perishock pauses.37,42-44
- Pulse checks and rhythm checks are common reasons for pauses in chest compressions that can reduce the CCF.45 Patient, environmental, and logistical factors can make pulse checks difficult to perform. Observational studies have shown that pulse checks can be prolonged greater than 10 seconds, further reducing the CCF.46
- Immediate resumption of chest compressions after a shock results in a shorter perishock pause and improves the overall hands-on time (CCF) during resuscitation. Two RCTs enrolling more than 1000 patients did not find any increase in survival when pausing CPR to analyze rhythm after defibrillation.47,48 Observational studies showed decreased ROSC when chest compressions are not resumed immediately after shock.38,49
- Chest compression depth begins to decrease after 90 to 120 seconds of CPR, although compression rates do not decrease significantly over that time window.50 A randomized trial using manikins found no difference in the percentage of high-quality compressions when rotating every 1 minute compared with every 2 minutes.51 Rotating the designated chest compressor every 2 minutes is sensible because this approach maintains chest compression quality and takes advantage of when CPR would ordinarily be paused for rhythm analysis.
- Keeping pauses for ventilations as short as possible helps to maintain high CCF and improve resuscitation outcomes.40,41,52 However, there is new evidence that ventilations delivered during the pause when using a 30:2 compression-to-ventilation ratio are often ineffective. In an analysis of the Resuscitation Outcomes Consortium data, investigators found that effective ventilations were delivered during fewer than half of the pauses. Importantly, when compared to the group for whom effective ventilations were provided more than 50% of the time, patients with a lower proportion of effective ventilations had worse rates of ROSC, survival to hospital discharge, and survival with favorable neurologic outcome.53
- Minimizing interruptions in CPR and maintaining a CCF of at least 60% has been associated with improved outcomes in a number of observational studies.39,40,52 A 2015 systematic review reported significant heterogeneity among studies, with some studies, but not all, reporting better rates of survival to hospital discharge associated with higher CCF.40,41,52 In 2 studies, higher CCF was associated with lower odds of survival.54,55 A 2020 retrospective review of the Resuscitation Outcomes Consortium found an association between higher CCF and ROSC, but not survival, among patients with a nonshockable rhythm and a CCF of at least 40%.39 Compression rate and depth and associated interventions such as defibrillation, airway management, and medications, are also important and may interact with CCF. While a CCF of 60% is considered a minimum target, high performance teams may achieve higher levels (eg: greater than 80%).
- Interruptions to CPR should only be done for interventions that are associated with improved outcomes. At this time, there are insufficient data and evidence to support or refute any recommendations about checking a pulse during CPR.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. During manual CPR, rescuers should perform chest compressions to a depth of at least 2 inches, or 5 cm, for an average adult while avoiding excessive chest compression depths (greater than 2.4 inches, or 6cm) |
| 2a | B-R | 2. During adult cardiac arrest, it is reasonable to use audiovisual feedback devices for real-time optimization of CPR performance. |
| 2a | B-NR | 3. For adults in cardiac arrest, it is reasonable for rescuers to perform chest compressions at a rate of 100 to 120/min. |
| 2a | C-LD | 4. It can be beneficial for rescuers to allow complete chest wall recoil for adults in cardiac arrest, such as by not leaning on the chest between compressions. |
| 2b | C-EO | 5. During adult cardiac arrest, it may be reasonable to perform chest compressions so that chest compression and recoil/relaxation times are approximately equal. |
Synopsis
Key components of high-quality CPR include minimizing interruptions, compressing at an optimal rate and depth, providing adequate chest recoil, and avoiding excessive ventilation.34,56,57 Although there are numerous retrospective observational studies, there is a paucity of prospective studies or randomized trials specifically examining CPR quality targets. Further, evidence suggests interactions between CPR components (eg, rate and depth) confound studying them in isolation. These limitations do not reduce the importance of these elements, rather they underscore the need for ongoing investigations into ideal CPR performance.
Recommendation-Specific Supportive Text
- A 2020 ILCOR scoping review56 identified 12 studies, including over 10 700 patients looking at chest compression depth. Several studies found improved survival to hospital discharge55,58,59 when compression depth was at least 5 cm, compared to less than 4 cm.58,59 Observational research has suggested reduced survival with chest compressions of excessive depth (greater than 6cm).58,59
- A 2024 ILCOR scoping review60 addressing real-time CPR audiovisual feedback for rescuers affirmed previous recommendations, however, with methodologic limitations of included studies.13 Many studies incorporated feedback devices into multifaceted quality improvement programs. A recent systematic review and meta-analysis showed that the use of audiovisual feedback devices was associated with improved rates of ROSC and survival to discharge from cardiac arrest, although not survival with favorable neurologic outcome.61 One randomized clinical trial of IHCA demonstrated an improvement of 25.6% in survival to hospital discharge by using audible feedback on compression depth and recoil.62
- A 2020 ILCOR scoping review identified 11 observational studies examining chest compression rate in adult patients in cardiac arrest.56 Three studies of over 13,700 patients57,63,64 suggested improved survival to hospital discharge with compression rates of 100 to 119/min, compared with lower or higher rates. One randomized trial found no difference in survival between chest compression rates of 100 and 120/min.65 In 1 study, ROSC was improved with higher rates (121–140/min) in comparison to 100 to 120/min (n=222)64; however, the writing group placed higher value on survival compared with ROSC when making this recommendation.
- Two observational studies provide mixed results for the effect of chest compression release velocity, with 1 study suggesting improved survival at rates greater than 400 mm/s,66,67 and the other suggesting no difference in outcomes.66 Porcine study data suggest decreased coronary perfusion with rescuers leaning on the chest.68
- The 2020 Guidelines recommended a 50% duty cycle, in which the time spent in compression and decompression was equal, mainly based on its perceived ease of being achieved in practice. In a clinical study in adults with out-of-hospital ventricular fibrillation (VF) arrest (of whom 43% survived to hospital discharge), the mean duty cycle observed during resuscitation was 39%.69 Although many animal studies have observed higher blood flows and better outcomes when the duty cycle was less than 50%, the optimal duty cycle is not known. Currently, there is insufficient evidence to warrant a change from the existing recommendation, which remains a knowledge gap that requires further investigation.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In adult patients in cardiac arrest without an advanced airway, it is reasonable to deliver breaths either by mouth-to-mouth, mouth-to-mask, or bag-mask ventilation. |
| 2a | C-LD | 2. When ventilating an adult patient in cardiac arrest, it is reasonable to give enough tidal volume to produce visible chest rise. |
| 2a | C-LD | 3. When delivering ventilations by bag mask for an adult in cardiac arrest, it is reasonable for 1 rescuer to use 2 hands to open the airway and seal the mask to the face while a second rescuer squeezes the bag. |
| 2b | C-LD | 4. When providing breaths to an adult in cardiac arrest without an advanced airway, it may be reasonable to give each breath over 1 s. |
| 3: Harm | C-LD | 5. When providing breaths to adult patients in cardiac arrest, rescuers should avoid hypoventilation (too few breaths or too little volume) or hyperventilation (too many breaths or too large a volume). |
Synopsis
Ventilation plays a critical role during CPR and is linked to better patient outcomes.1-3 However, providing adequate ventilation during CPR without an advanced airway can be challenging, particularly when using a bag-mask device. With bag-mask ventilation, there is a risk of an inadequate seal between the mask and the face or unintended airflow into the esophagus instead of the lungs. Because measuring ventilation volume can be difficult with the available equipment, rescuers can monitor for adequate ventilation by observing chest rise. Performing chest compressions with passive oxygen delivery does not ensure sufficient ventilation. Furthermore, chest compressions can reduce functional residual capacity, impair lung compliance due to intermittent airway collapse and atelectasis, and trigger a series of complications, including lung injury and decreased cardiac output.4-9 Recent studies show that rescuers often fail to deliver ventilation in accordance with guidelines.2,10,11 In contrast, CPR with both effective ventilation and chest compressions (with lung inflation in more than 50% of the pauses during 30:2 CPR) was associated with improved outcomes.1-3 These findings highlight the importance of adequate ventilation to optimize patient outcome.
Recommendation-Specific Supportive Text
- Both mouth-to-mouth breaths and bag-mask ventilation provide oxygen and ventilation to the person in cardiac arrest.12 Oxygen delivery can be increased by adding supplemental oxygen flow to bag-mask devices or certain pocket masks. To provide mouth-to-mouth breaths, open the person’s airway, pinch the person’s nose, create an airtight seal around the mouth, and provide a breath. When using a barrier device, use of mouth-to-pocket mask provides more effective breaths than mouth-to-face shield.13
- Ventilation during chest compressions presents a challenge, particularly because equipment to monitor tidal volumes or respiratory rates is typically unavailable in the prehospital setting. However, observing chest rise and fall remains a reliable method for assessing ventilation. One study of intubated adults found that a tidal volume of 362 to 406 mL (approximately 5–7 mL/kg for the average adult) was necessary to achieve visible chest rise.14 While chest rise can help guide ventilation during standard 30:2 CPR, where compressions are paused for ventilation, it is not possible to monitor chest rise during continuous chest compression CPR. With bag-mask ventilation, air leakage between the mask and the face often results in inadequate tidal volume, particularly when using a pediatric-size bag.10 In comparison, an adult-size bag has been associated with a higher rate of ROSC in adult cardiac arrest.11
- Bag-mask ventilation is a challenging skill that requires considerable practice for competency. It often fails to provide adequate ventilation, due to air leakage between the mask and the face or an incompletely opened airway.2,15 Bag-mask ventilation is most effective when provided by 2 trained and experienced rescuers; 1 rescuer opens the airway and seals the mask to the face with both hands while the other rescuer (who might also be the chest compressor) squeezes the bag during the pauses in chest compression during 30:2 CPR. Both rescuers watch for visible chest rise and fall to confirm proper ventilation. The 2-handed mask technique with jaw thrust has been shown to be superior to a 1-handedtechnique that is often referred to as the E-C clamp.16,17 The E-C clamp uses the index finger and thumb in the shape of a C to create a seal over the nose and mouth while the remaining fingers are placed along the lower edge of the jaw in an E formation to elevate the mandible and open the airway. In cases where only 1 rescuer is available, a 2-handed mouth-to-mask ventilation technique can be used during 30:2 CPR, but if a bag mask is used a 1-handed technique to seal the mask and open the airway is necessary.
- Taking a regular rather than a deep breath may help prevent the rescuer from getting dizzy or lightheaded when giving mouth-to-mouth or mouth-to-mask breaths and prevents overinflation of the person’s lungs. The recommendation for using a 1-second inspiratory time is to keep the pauses in chest compressions as brief as possible during 30:2 CPR.18
- Excessive ventilation can cause gastric inflation, regurgitation, aspiration, and decreased cardiac output.1-3 Too little ventilation also is harmful and is associated with decreased survival.1-3
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. It is reasonable for a rescuer to use mouth-to-nose ventilation if providing breaths through the adult’s mouth is impossible or impractical. |
| 2b | C-EO | 2. For an adult with a tracheal stoma who requires breaths, either mouth-to-stoma or face mask–to–stoma ventilation may be reasonable. |
Synopsis
While pocket mask and bag-mask ventilation use both the oral and nasal passages, the performance of mouth-to-mouth breaths requires a seal between the rescuer and the mouth of the person in cardiac arrest. If certain anatomic, pathologic, and traumatic conditions prevent ventilation via the mouth, use of the nasal passage is an acceptable alternative.
Individuals who have a tracheal stoma may experience respiratory or cardiac arrest necessitating breaths. If ventilations cannot be performed via the nose or mouth, the rescuer can use the tracheal stoma to provide breaths with the mouth or a face mask. The management of a transtracheal artificial airway (eg, tracheostomy) is beyond the scope of these guidelines. No new evidence was identified on this topic during the 2025 evidence review.
Recommendation-Specific Supportive Text
- Mouth-to-nose ventilation may be necessary if ventilation through the person’s mouth is impossible because of trauma, positioning, or difficulty obtaining a seal. A case series suggests that mouth-to-nose ventilation in adults is feasible, safe, and effective.19
- Effective ventilation of an adult patient with a tracheal stoma may require ventilation through the stoma, either by using mouth-to-stoma breaths or by use of a bag-mask technique that creates a tight seal over the stoma with a round, pediatric face mask. There is no published evidence on the safety, effectiveness, or feasibility of mouth-to-stoma ventilation. One study of patients with laryngectomies showed that a pediatric face mask created a better peristomal seal than a standard ventilation mask.20
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. For an adult with spontaneous circulation (ie, strong and easily palpable pulses) and respiratory arrest, the health care professional should provide ventilations. |
| 2a | C-LD | 2. When providing ventilations for an adult patient with respiratory arrest, it is reasonable for the health care professional to give 1 ventilation every 6 s, or 10 breaths/min, with each ventilation creating visible chest rise. |
Synopsis
Ventilation is essential for oxygen delivery and carbon dioxide elimination for a person with a pulse who is not breathing or not breathing effectively. If effective ventilations are not provided quickly to an apneic patient, high oxygen-consuming organs such as the heart and brain may sustain irreversible injury. Examples of such conditions include opioid overdose and ROSC after cardiac arrest. In people with a pulse, after only 90 seconds of apnea, oxygen saturation can drop to dangerously low levels.21 Oxygen saturation can be restored rapidly with effective ventilation.22
Recommendation-Specific Supportive Text
- A study of 14 patients who underwent general anesthesia while breathing room air found that oxygen saturation dropped to dangerously low levels within 90 seconds after onset of apnea (mean oxygen saturation, 62.1%; range, 43.9%–81.9%).21 Respiratory arrest and hypoxia can quickly deteriorate into cardiac arrest if not effectively and aggressively managed with appropriate oxygenation and ventilation. In the recently updated 2024 Guidelines for resuscitation from drowning, the use of in-water breathing support before cardiac arrest was associated with improved outcomes compared to initiation of CPR after rescue from the water.23
- A randomized trial in critically ill apneic adult patients compared bag-mask ventilation with no ventilation and found that bag-mask ventilation at a rate of 10 breaths per minute compared with no ventilation decreased the incidence of severe hypoxic events before intubation.22 The tidal volume used was enough to cause visible chest rise. While little data exist to guide the exact frequency of ventilations required to produce normocapnia (normal ventilation) during cardiac arrest, an in-hospital study examining abnormal ventilation found that any hypocapnia (too much ventilation), severe hypercapnia (too little ventilation), and swings in both hypocapnia and hypercapnia (inconsistent ventilation) were associated with a higher rate of 1-month unfavorable neurological outcome compared with mild hypercapnia.24
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. It is reasonable for lay rescuers and health care professionals to perform CPR with cycles of 30 compressions followed by 2 breaths before placement of an advanced airway (supraglottic airway or endotracheal tube). |
| 2b | C-LD | 2. For adults in cardiac arrest without an advanced airway, health care professionals may consider the use of continuous chest compressions with asynchronous breaths. |
Synopsis
Several strategies for performing adult CPR have been described in the literature. The 2 main strategies include chest compressions with interrupted ventilation pauses (eg, 30 compressions for every 2 ventilations) and continuous chest compressions with or without asynchronous ventilations.
Recommendation-Specific Supportive Text
1 and 2. The majority of studies report no difference in patient outcomes between continuous chest compressions and interrupted CPR with ventilation pauses.1-5 A systematic review and meta-analysis of 4 RCTs of nonasphyxial cardiac arrests reported lower survival to hospital discharge when lay rescuers administered interrupted CPR compared with continuous compressions (100 compressions per minute), but the compression-to-ventilation ratio in 3 of the studies was 15:2.6 CPR performed by prehospital health care professionals was associated with increased ROSC, survival to admission, and favorable neurologic outcome with 30:2 CPR compared with continuous chest compressions with asynchronous ventilations. No difference was found in survival to hospital discharge.6 A retrospective analysis of the Resuscitation Outcomes Consortium Continuous Chest Compressions trial analyzed 26 810 adults with nontraumatic OHCA without an advanced airway. When performed as intended (per protocol), continuous chest compressions with asynchronous ventilations by EMS were associated with lower odds of survival compared with 30:2 CPR.7 After the imputation of missing outcomes, the overall difference in the survival rate between the treatment groups in the effectiveness population was not significant.
Importantly, the use of continuous chest compressions with asynchronous breaths does not allow the rescuer to monitor the adequacy of ventilation during CPR because chest rise cannot be observed. Recent evidence has shown that ventilations are often not provided with sufficient volume to make the chest rise during resuscitation.8 Because it is not possible to evaluate ventilations during continuous chest compressions, value was placed on the ability to monitor chest rise during 30:2 CPR.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. It is reasonable to place defibrillation paddles or pads on the exposed chest in an anterolateral or anteroposterior position, and to use a paddle or pad electrode diameter more than 8 cm for adults in cardiac arrest. |
| 2b | C-EO | 2. When placing pads for defibrillation for an adult in cardiac arrest, it might be reasonable to adjust the position of a bra instead of removing it. |
Synopsis
The use of paddles or pads to perform defibrillation requires direct contact with the skin, and the use of pregelled pads or electrolyte gel can further improve conduction of electricity to the myocardium. Pad placement is important to ensure that the vector of energy delivery is directed toward the myocardium, including the left ventricle where VF often originates. If using an anterior-lateral pad placement, it is important that the lateral pad is placed in the mid-axilla and not too anteriorly (Figure 7). The size of the pad or paddle affects impedance, thus larger paddles or pads are appropriate for adults. The need to apply pads or paddles directly to the bare chest may be a contributing factor to the observations that females experience significantly lower rates of public access defibrillation compared to males.1,2 The option to adjust rather than remove a bra could mitigate factors such as discomfort with exposing a woman’s chest or fear of accusations of inappropriate touching or sexual assault.3
Recommendation-Specific Supportive Text
- Anterolateral, anteroposterior, anterior-left infrascapular, and anterior-right infrascapular electrode placements are comparably effective for treating supraventricular and ventricular arrhythmias.4-8 A recent before-and-after study of 207 patients (1023 shocks) found no difference in shock success between anterior-lateral versus anterior-posterior pad placement (82.1% versus 82.2%).9 A second study found increased rates of ROSC with anterior-posterior pad positioning compared to anterior-lateral (odds ratio, 2.64; 95% CI, 1.50–4.65), but no difference in rates of survival to hospital discharge or favorable neurologic outcome.10 Due to the potential for significant risk of bias, the writing group felt that more research is required to confirm these findings. A larger pad/paddle size (within the limits of 8–12 cm in diameter) lowers transthoracic impedance. Self-adhesive pads have largely replaced defibrillation paddles in clinical practice.11,12
- A 2024 ILCOR scoping review identified 3 studies addressing the removal of clothing, comprising 1 peer-reviewed simulation study and 2 conference abstracts. The review concluded that there was insufficient evidence to support the routine removal of a bra, and it may be possible to place pads directly on the skin by adjusting the bra's positioning rather than removing it. The single peer-reviewed simulation study demonstrated13 a swine model, published as a conference abstract, and reported a 100% first-shock success rate even when self-adhering AED pads were applied directly over the metal underwire of a bra.14 No adverse events such as arcing, current redirection, or burning of the bra or the pig’s skin were observed in the study.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. For adults in cardiac arrest, CPR is recommended until a defibrillator or AED is applied. |
| 2a | B-R | 2. In unmonitored adult cardiac arrest, it is reasonable to provide a brief period of CPR while a defibrillator is being obtained and readied for use. |
| 2a | C-LD | 3. For adults in cardiac arrest, immediate defibrillation is reasonable for witnessed or monitored VF/pulseless ventricular tachycardia when a defibrillator is already applied or immediately available. |
Synopsis
Early defibrillation is essential for survival when sudden cardiac arrest results from VF or pulseless ventricular tachycardia (pVT). Defibrillation is most effective when delivered promptly after the onset of VF/pVT, especially if the time to shock is minimal.15 However, when VF/pVT persists for a longer duration, the heart’s energy reserves may become depleted, reducing defibrillation effectiveness unless preceded by a period of CPR to restore these reserves before rhythm analysis. Minimizing the interruption in CPR around the time of shock delivery, or the perishock period, is also crucial to improving patient outcomes.16 Defibrillation success refers to termination of VF/pVT, although this may not result in a perfusing rhythm.
Recommendation-Specific Supportive Text
- High-quality CPR is the single most critical intervention for a patient in cardiac arrest. Performing CPR until a defibrillator or AED is available and applied, and ensuring minimal interruptions to chest compressions in the perishock period, is associated with improved defibrillation success and patient outcomes.17-23 A study from the ARREST (Amsterdam Resuscitation Studies) registry of patients with witnessed out-of-hospital arrest and initial rhythm of VF showed that first-shock defibrillation success rates were 93% when first shock occurred within 6 minutes, but decreased to 75% if first shock was delayed to more than 16 minutes. Every minute of delay to first shock was associated with a 6% decreased probability of survival to discharge.24
- When VF/pVT has been present for more than a few minutes, myocardial reserves of oxygen and other energy substrates are rapidly depleted.25 If replenished by a period of CPR before shock, defibrillation success improves significantly.17-20 Studies comparing short (approximately 30 seconds) with prolonged (up to 3 minutes) periods of CPR preceding the initial rhythm analysis show no difference in defibrillation outcomes; therefore, a brief period of CPR while the defibrillator is readied for use may be sufficient in unmonitored cardiac arrest.21-23 Even in monitored arrests, it can take time to attach defibrillator pads, power on the defibrillator, and charge the capacitor before shock delivery, during which rescuers can continue CPR.
- Early defibrillation improves outcomes from cardiac arrest.26-28 When VF is of short duration, myocardial reserves of oxygen and other energy substrates are likely to remain intact, and the rhythm is most responsive to defibrillation.25,29 Thus, if the onset of VF is monitored or witnessed while a defibrillator is already applied, or there is immediate access to a defibrillator, defibrillation can be performed immediately.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For adults with severe FBAO, repeated cycles of 5 back blows (slaps) followed by 5 abdominal thrusts should be performed until the object is expelled or the person becomes unresponsive. |
| 1 | C-EO | 2. Rescuers should activate the emergency response system for adults with severe FBAO. |
| 1 | C-EO | 3. For adults with mild FBAO, the person should be allowed to clear the airway by coughing while being observed for signs of severe airway obstruction. |
Synopsis
FBAO is a common medical emergency and one of the leading causes of accidental death in the United States, with over 5000 cases in 2021.1 Most adult FBAO cases are due to food, while FBAOs in pediatrics are commonly caused by both food and nonfood material. Cases of FBAO are often classified as mild or severe depending on the degree of obstruction. Mild, or partial, airway obstructions often present with coughing and difficulty breathing. These cases can often be relieved spontaneously with coughing, however, can progress to complete airway obstructions. Severe, or complete, airway obstructions present with weak or absent coughing, inability to speak, changes in color (cyanosis), and altered level of consciousness which can rapidly progress to unconsciousness, apnea, and cardiac arrest if the foreign body is not removed. Longer airway obstruction time is associated with high morbidity and mortality.2 Prompt performance of appropriate interventions to relieve the FBAO by lay rescuers has been associated with improved survival and favorable neurological outcome.3 Additionally, activation of emergency medical services is important to provide additional techniques for FBAO removal and provide transport to a hospital for further care, regardless of removal of the foreign body.
Recommendation-Specific Supportive Text
- There are no RCTs comparing the efficacy or safety of different interventions for FBAO, and limited evidence about the most effective sequence of FBAO interventions in both health care and non–health care settings.3,4 A cohort study5 of 709 persons with FBAO showed back blows were associated with improved rates of FBAO relief and fewer injuries compared to abdominal thrusts. In addition, case reports have described fatal injuries from abdominal thrusts including aortic dissection and gastric rupture.4,6,7 The recommendation for alternating sets of 5 back blows and 5 abdominal thrusts is based on the value of consistency with existing infant and pediatric guidelines that use this approach.8,9
- Previous observational studies have demonstrated that patients with FBAO commonly require multiple interventions before the foreign body is successfully removed. Severe FBAO cases often require intervention by prehospital care personnel or evaluation and treatment at a hospital.3,5 Thus, promptly activating the emergency medical response system is essential for severe FBAO.
- Coughing can produce high airway pressures,10,11 making it effective in clearing partial obstructions. Mild FBAO can quickly progress; therefore, monitoring for signs of severe obstruction is crucial.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. If adults with severe FBAO become unresponsive, rescuers should start CPR, beginning with chest compressions, and activate the emergency response system if no one has done so. |
| 1 | C-EO | 2. For adults with FBAO receiving CPR, rescuers should remove any visible foreign body when opening the airway to provide breaths. |
| 3: Harm | C-LD | 3. Blind finger sweeps should not be performed for adults with FBAO. |
Synopsis
When a severe airway obstruction leads to unconsciousness, back blows (slaps) and abdominal thrusts become impractical, and cardiac arrest is imminent. Chest compressions can provide sufficient airway pressure to expel a foreign body.12,13
Recommendation-Specific Supportive Text
- Once a patient is unconscious, observational data support immediate provision of CPR starting with chest compressions, which is associated with favorable neurologic outcome, regardless of whether or not the patient has a pulse.14 As previously noted, there is low risk of injury from performance of chest compressions for patients who are not in cardiac arrest.8
- Removal of a visible foreign body will help to avoid reobstruction that could occur with ventilations. There is, however, no evidence that providing ventilations in the presence of a foreign body will make the obstruction worse, and theoretically could alleviate a complete obstruction by forcing the obstruction into a mainstem bronchi.
- Observational data suggest that when a foreign body is not visible, the risk associated with performing a “blind” finger sweep and worsening an airway obstruction outweighs any potential benefit.4
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. If the rescuer is unable to encircle the person’s abdomen, repeated cycles of 5 back blows (slaps) followed by 5 chest thrusts should be used for adults with severe FBAO until the object is expelled or the person becomes unresponsive. |
| 1 | C-EO | 2. For adults in the late stages of pregnancy with severe FBAO, repeated cycles of 5 back blows (slaps) followed by 5 chest thrusts should be used until the object is expelled or the person becomes unresponsive. |
| 2b | C-LD | 3. Effectiveness and safety of suction-based airway clearance devices have not been established in adults with FBAO. There is insufficient evidence to make a recommendation. |
Synopsis
There are several special circumstances in which effective abdominal thrusts are either impossible to perform or inappropriate (eg, when rescuers cannot encircle the patient's abdomen, patient is in a wheelchair, or in the late stages of pregnancy). Evidence on FBAO management for these situations is limited.
Recommendation-Specific Supportive Text
1 and 2. There are no high-quality data to support recommendations about FBAO in special circumstances, such as when rescuers cannot encircle the patient's abdomen or the patient is in late-stage pregnancy. It may be impractical for rescuers to provide abdominal thrusts in these situations. Chest thrusts can be used in these situations to provide sufficient pressure to expel the foreign body.
3. Several observational studies15,16 describe the use of portable nonpowered suction devices. However, there is a lack of evidence suggesting the superiority of these devices compared with standard techniques such as back blows or abdominal thrusts, or data establishing safety of the devices.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. CPR for adults with obesity who are in cardiac arrest should be provided by using the same techniques as for the nonobese patient. |
| 2a | C-LD | 2. In adults with obesity who are in cardiac arrest, it is reasonable for rescuers to consider the impact of moving the patient to a firm surface on delays in initiation of chest compressions. |
| 2b | C-EO | 3. It may be reasonable for rescuers to increase the force of compressions to achieve adequate depth for adult patients with obesity who are in cardiac arrest. |
| 3: No Benefit | C-LD | 4. For adults with obesity, the use of a partial backboard (or “CPR board”) during IHCA does not provide benefit over performing chest compressions on a hospital mattress. |
Synopsis
Obesity affects more than 40% of adults in the United States and is a significant risk factor for sudden cardiac arrest. In an observational study of cardiac arrest survivors with significant coronary artery disease, obesity was associated with high risk of mortality and unfavorable neurologic recovery.1 Cardiac arrest management, including performing effective chest compressions, can be difficult in patients with obesity due to increased chest wall thickness, requiring greater force to achieve the recommended depth.2 The physical demands of performing CPR on an adult patient with obesity can lead to faster rescuer fatigue, highlighting the need for close monitoring to switch rescuers.
Recommendation-Specific Supportive Text
- A 2024 ILCOR scoping review included 34 observational studies evaluating cardiac arrest in adult patients with obesity and found no evidence to support changes from standard CPR. One challenge to this review was that the reported patient outcomes, such as neurological outcomes, survival to hospital discharge, long-term survival, and ROSC, varied and were inconsistently measured across studies.3 There were no differences between patients with obesity and patients without obesity in terms of shock success,4 percentage of shocks delivered in less than 2 minutes,5,6 or VF/pVT termination.6
- Firm surfaces improve the likelihood of adequate chest compressions. Use of bed manufacturer “CPR mode” in-hospital or a firm mattress can improve chest compression depth.2,7 During OHCA in which an adult patient with obesity is found in a bed, the time required to move the patient must be carefully considered to avoid delays in chest compression initiation.
- Rescuers may need to increase force of compressions to achieve adequate depth of compressions when compressions are performed on inflated mattresses or for adults with obesity.2
- Use of a partial backboard (or “CPR board”) in obese manikins does not improve likelihood of achieving adequate chest compressions compared with the hospital mattress alone.8 As rapid initiation of high-quality CPR improves outcomes from cardiac arrest, initiation of chest compressions for the patient with obesity while on the hospital mattress can help to minimize delays to CPR initiation.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. In adult cardiac arrest, the use of mechanical CPR devices may be considered in specific settings where the delivery of high-quality manual compressions may be challenging or dangerous for the health care professional, as long as rescuers strictly limit interruptions in CPR during deployment and removal of the device. |
| 2a | C-LD | 2. For single rescuers, performing compressions from “over-the-head” (OTH) of the patient may be considered in adult cardiac arrest. |
| 2b | C-LD | 3. The effectiveness of heel/foot compressions is not well established in adult cardiac arrest. |
| 3: No Benefit | B-R | 4. The routine use of mechanical CPR devices is not recommended in adult cardiac arrest. |
Synopsis
Alternatives to conventional CPR include mechanical CPR or alternative rescuer positions for use in special circumstances. Mechanical CPR can be divided into 2 types: a load-distributing compression band that compresses the thorax circumferentially or a pneumatic piston device which anteriorly to posteriorly compresses the chest. Use of these devices or techniques requires specialized equipment and additional training to minimize interruptions in CPR. Special circumstances such as during patient transport, search and rescue, or resuscitation in a confined space may support alternative methods of CPR where the rescuer is not lateral to the patient. One alternative position has been termed “over-the-head” compressions where rescuers are kneeling with the patient’s head between their knees and the rescuer is reaching down toward the sternum to perform chest compressions, with or without ventilations. Another novel alternative method includes a rescuer performing chest compressions by using their heel or foot. These alternative CPR methods are considerations when patients are in unusual locations or when resuscitation must take place under special circumstances.
Recommendation-Specific Supportive Text
- Individual emergency medical response agencies must weigh the potential benefits of mechanical CPR devices to logistical factors such as transport times, safety of crew, and number of personnel available for chest compressions against potential drawbacks such as interruptions in chest compressions related to application. Examples of scenarios for consideration of mechanical CPR use include the potential to improve CPR quality during patient transport, logistical constraints that may be impractical to perform manual CPR or may impact rescuer safety, prolonged resuscitations with limitations in the number of individuals for manual CPR, or a significant risk of infectious disease transmission.
- Single rescuers may perform effective chest compressions by compressing the chest from above the shoulders of the patient, known as over-the-head CPR. In this scenario, rescuers are positioned such that they are kneeling with the patient’s head between their knees and reaching down toward the sternum to perform chest compressions with or without ventilations. Multiple small- to moderate-sized manikin RCTs have shown no difference between chest compression quality including depth and recoil in over-the-head CPR compared to lateral CPR,1-5 while several studies found improved chest compression quality.6-8 Ventilation rates were improved in some of the over-the-head CPR studies while others showed no difference in ventilation quality or increased tidal volume delivery.4,6,7 Many of these studies were designed for consideration in special circumstances such as avalanche patients or confined spaces such as on the back of a moving boat.2,4 Multiple studies showed that standard dual-rescuer CPR, in which 1 rescuer performed ventilations at the head of the patient and the second rescuer performed CPR lateral to the patient, had improved chest compression quality compared to a single rescuer performing over-the-head chest compressions.7
- Limited observational studies showed improved duration of chest compression cycles and no difference in chest compression depth when compressions are delivered using the foot or heel in comparison to conventional hands-only CPR9,10 One simulation-based randomized trial showed decreased chest compression quality when rescuers performed compressions by using their heel versus conventional CPR.11 As data are significantly limited and show conflicting results, recommendations for or against the use of this technique cannot be provided.
- Several large RCTs demonstrated no improvement in survival with the use of mechanical CPR devices.12-16 This is further supported by a recent ILCOR systematic review.17 The Prehospital Randomised Assessment of a Mechanical Compression Device in Out-of-Hospital Cardiac Arrest (PARAMEDIC) trial found no survival benefit at 30 days.13,18 and worse neurological outcome at 30 days, 3 months, and 12 months with the use of mechanical devices.13,19,20 However, neurologic outcomes at 3 months and 12 months were significantly limited by loss to follow-up in greater than 40% of patients.20 A secondary analysis of the primary study population in the AutoPulse Assisted Prehospital International Resuscitation (ASPIRE) trial found decreased 4-hour survival with mechanical CPR, possibly associated with a delayed time to first shock, and the study was terminated due to potential harm in the mechanical group.12 Two small observational studies showed increased rib fractures and sternal fractures with mechanical CPR use.21,22 However, this finding has not been replicated in the published RCTs.13,19,20
The American Heart Association requests that this document be cited as follows: Kleinman ME, Buick JE, Huber N, Idris AH, Levy M, Morgan SG, Nassal MJ, Neth MR, Norii T, Nunnally ME, Rodriguez AJ, Walsh BK, Drennan IR. Part 7: adult basic life support: 2025 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2025;152(suppl 2):S448–S478. doi: 10.1161/CIR.0000000000001369
- Monica E. Kleinman, MD, Chair
- Jason E. Buick, MSc, CCPf
- Nicole Huber, PA-C
- Ahamed H. Idris, MD
- Michael Levy, MD
- Sean G. Morgan, MD
- Michelle MJ Nassal, MD, PhD
- Matthew R. Neth, MD
- Tatsuya Norii, MD
- Mark E. Nunnally, MD
- Amber R. Rodriguez, PhD
- Brian K. Walsh, PhD
- Ian R. Drennan, ACP, PhD, Vice Chair
Open table in a new window.
Open table in a new window.
 Login
Login