Part 5: Neonatal Resuscitation
Abstract
The guidelines in this document from the American Heart Association and the American Academy of Pediatrics focus upon optimal care of the newborn infant, including those who are proceeding to a normal transition from the fluid-filled uterine environment to birth. Newborn infants who are proceeding to normal transition can benefit from deferred cord clamping for at least 60 seconds in most instances, skin-to-skin with their parent soon after birth, and appropriate assistance with thermoregulation. Some newborn infants require assistance during transition, with interventions ranging from warming and tactile stimulation to advanced airway management, assisted ventilation, oxygen therapy, intravascular access, epinephrine, and volume expansion. In this context, individuals, teams, and health care settings that care for newborn infants should be prepared and have access to appropriate training and resources for neonatal resuscitation. The newborn chain of care provides guidance on considerations that may lead to optimal outcomes for newborn infants starting from prenatal care to recovery and follow-up.
Top 10 Take-Home Messages
- The newborn chain of care starts with prenatal care and extends to recovery and appropriate follow-up in the postnatal period to ensure optimal short- and long-term health for the infant and family
- Newborn resuscitation requires anticipation and preparation by clinicians who train individually and as teams.
- Most newborn infants do not require resuscitation. They can be evaluated and monitored during deferred cord clamping for 60 seconds or more and can maintain skin-to-skin contact with their parents after birth.
- Effective ventilation of the lungs, as indicated by an increasing heart rate, is the priority in newborn infants who need resuscitation.
- Ventilation corrective steps, including the use of an alternative airway (laryngeal mask or endotracheal intubation), may be required if the heart rate does not rise with assisted ventilation with a face mask.
- Monitoring temperature during resuscitation helps to avoid hypothermia and hyperthermia, both of which are associated with adverse outcomes.
- Pulse oximetry is used to guide oxygen therapy and meet oxygen saturation target ranges.
- Chest compressions are indicated if the heart rate remains less than 60 beats per minute after appropriate ventilation corrective steps, which preferably include endotracheal intubation.
- If the heart rate remains less than 60 beats per minute after chest compressions, epinephrine is indicated, preferably via an intravascular route. Endotracheal epinephrine may be considered while vascular access is being obtained.
- If all steps of resuscitation are effectively completed and there is no heart rate detected by 20 minutes, redirection of care may be discussed with the team and family.
Preamble
Approximately 5% to 10% of newborn infants need help to begin breathing at birth, and approximately 1% need advanced resuscitative measures to restore cardiorespiratory function.1-3 The neonatal mortality rate in the United States and Canada has decreased from nearly 20 per 1000 live births in the 1960s to the current rate of approximately 3.5 per 1000 live births.4,5 Yet there remain opportunities for improvement and increasing access to quality care with disparities in neonatal mortality by region and disparities in access to care.6-10 The inability of newborn infants to establish and sustain adequate or spontaneous respiration contributes significantly to early deaths and the burden of adverse neurodevelopmental outcomes among survivors. Effective and timely resuscitation at birth could therefore improve neonatal outcomes and reduce health disparities.
Successful neonatal resuscitation efforts depend on time-critical actions that must occur in rapid succession to maximize the chances of intact survival. The International Liaison Committee on Resuscitation (ILCOR) Formula for Survival emphasizes 3 essential components for good resuscitation outcomes: guidelines based on sound resuscitation science, effective education of health care professionals in resuscitation, and implementation of effective and timely resuscitation.11 The 2025 neonatal resuscitation guidelines contain recommendations based on the best available resuscitation science for the most impactful steps to perform during delivery and in the neonatal period. In addition, specific recommendations regarding systems of care and training of resuscitation are provided in the respective guidelines, Parts 412 and 12.13 The 2025 guidelines also introduces the Newborn Chain of Care (Figure 1), which addresses the overall context in which neonatal resuscitation occurs and how systems of care may impact newborn infant outcomes.
Newborn Chain of Care
The Newborn Chain of Care (Figure 1) provides a framework for considering essential elements of the health care system relating to neonatal health. A robust newborn chain of care has the potential to enhance health during the perinatal and neonatal periods and long-term outcomes. Each link in the chain is described in detail here.
Link 1: Prevention
The health of the pregnant person has a direct impact on the health of the newborn infant. Optimal care during pregnancy can help prevent or mitigate risks to the developing and growing fetus. Antenatal care includes individualized risk assessment, psychosocial support, education, and evaluation of personal and societal factors to achieve optimal health outcomes. Comprehensive care encompasses counseling on nutrition, medication, substance use, potential environmental exposures, safety, mental health, and self-care. Comprehensive care also includes early estimation of gestational age and ongoing screening, prevention measures, and interventions for conditions that may impact the health of the pregnant person or the fetus. Screening modalities include review of symptoms, measurements of blood pressure and fundal height, laboratory tests, ultrasonography, and monitoring of fetal heart rate, when indicated.14
Link 2: Recognition and Activation
Approximately 5% to 10% of newborn infants require assistance to breathe after birth. The need for assistance must be anticipated and recognized to reduce the risk of poor outcomes. Risk assessments evaluate factors present during pregnancy and labor that can help health care professionals identify newborn infants who are more likely to require resuscitation. When risk factors are present, an appropriate team should be mobilized to assist with resuscitation. In some cases, this may involve transferring the pregnant person to a higher level of care to a specialized hospital. When appropriate, based on anticipated risk, a qualified team with advanced resuscitation skills should be present at the time of birth. Refer to Anticipation and Preparation for Resuscitation.
Link 3: Initial Steps
Initial care at birth consists of lifesaving measures essential for all newborn infants, including those who are well and those who require respiratory support or advanced resuscitation. Steps to maintain normal temperature include maintaining a warm environment, promptly drying the infant, and skin-to-skin contact with the parent, keeping the infant’s body and head covered while leaving the mouth and nose visible. Newborn infants receiving resuscitation may require alternative methods such as covering the body with plastic wrap or a bag and providing supplemental heat from a radiant warmer or thermal mattress. Umbilical cord clamping can be deferred for at least 60 seconds while performing the initial steps of drying, evaluating breathing, providing tactile stimulation if needed to support breathing, and clearing the airway if there is evidence of obstruction. Whenever possible, skin-to-skin contact is maintained with monitoring of breathing and temperature for at least 1 hour to support early initiation of breastfeeding. Refer to Umbilical Cord Management and Initial Steps.
Link 4: Ventilation
Newborn infants who do not breathe within the first 60 seconds after birth or have a heart rate less than 100 per minute despite initial steps should receive assisted ventilation. Delaying ventilatory support in newborn infants increases the risk of death. A face mask or laryngeal mask (supraglottic airway) can be used to provide initial ventilation. Leak and obstruction are common during face mask ventilation and are important to address because they prevent effective ventilation. A rise in heart rate is the primary indicator of effective ventilation. If the newborn infant’s heart rate remains less than 100/min and does not increase after ventilation, inserting an alternative airway, such as a laryngeal mask or an endotracheal tube often improves effectiveness of ventilation. For spontaneously breathing preterm infants who need respiratory support, continuous positive airway pressure (CPAP) is a form of noninvasive respiratory support that helps establish and maintain functional residual capacity after birth. Refer to Ventilation and Continuous Positive Airway Pressure.
Link 5: Advanced Resuscitation
Advanced neonatal resuscitation requires a comprehensive approach to address a life-threatening situation in newborn infants. If initial steps and ventilation with an alternative airway do not improve the heart rate, then providing advanced interventions, establishing intravascular access, and administering of epinephrine are crucial. Chest compressions are initiated when the newborn infant’s heart rate remains less than 60/min despite adequate ventilation. Chest compressions aim to restore cardiac output. Concurrently, epinephrine is administered to augment perfusion to vital organs. Establishing intravascular access via umbilical venous catheterization or an intraosseous (IO) device is imperative for swift medication delivery and fluid resuscitation. Through the meticulous execution of these advanced interventions, health care professionals aim to optimize outcomes in newborn infants. Refer to Chest Compressions, Vascular Access During Resuscitation, Epinephrine, and Volume Expansion.
Link 6: Postnatal Care
Most healthy newborn infants can remain with their parent, prioritizing bonding, breastfeeding, and thermoregulation immediately following birth. These steps contribute to the infant’s health and stability. Newborn infants who require resuscitation beyond initial steps are at risk for abnormal transition to extrauterine life and require further observation and monitoring. Transitional issues can include respiratory compromise, hypoglycemia, or hypothermia, among others. Monitoring should include assessment of respiratory effort and oxygenation, heart rate, temperature, and blood glucose. The duration of monitoring depends on the newborn infant’s status and transition to postnatal stability. Some newborn infants may need interhospital transfer to a higher level of care during the postnatal period. Refer to Postresuscitation Care.
Link 7: Recovery
Ensuring appropriate primary care follow-up for all newborn infants after birth, as well as their parents, is crucial for promoting short- and long-term health. Appropriate follow-up, education, and support for families are even more critical for those infants who require specialized care in the neonatal period. Infants who are born very preterm, have congenital anomalies, require resuscitation, or experience neonatal complications will require specialized services post-discharge to effectively address their unique needs. A safe and optimal transition from hospital to home may involve medical therapies such as tube feeding, cardiorespiratory monitoring, and portable oxygen, as well as arrangements for specialized services including developmental follow-up and early intervention services. Close communication and collaboration with the outpatient healthcare team, including social service agencies, can promote the health of infants at risk of developmental difficulties, particularly for those families who may have challenges navigating the health care system.15
Scope of the Guidelines
This guideline is designed for North American health care professionals seeking an up-to-date summary for clinical care, as well as for those looking for more in-depth information on resuscitation science and gaps in current knowledge. The science of neonatal resuscitation applies to infants at birth and in the days after birth. In circumstances of altered or impaired transition, effective neonatal resuscitation reduces the risk of mortality and morbidity. Even healthy babies who breathe well after birth benefit from the facilitation of normal transition, including appropriate umbilical cord management and thermal protection with skin-to-skin care.
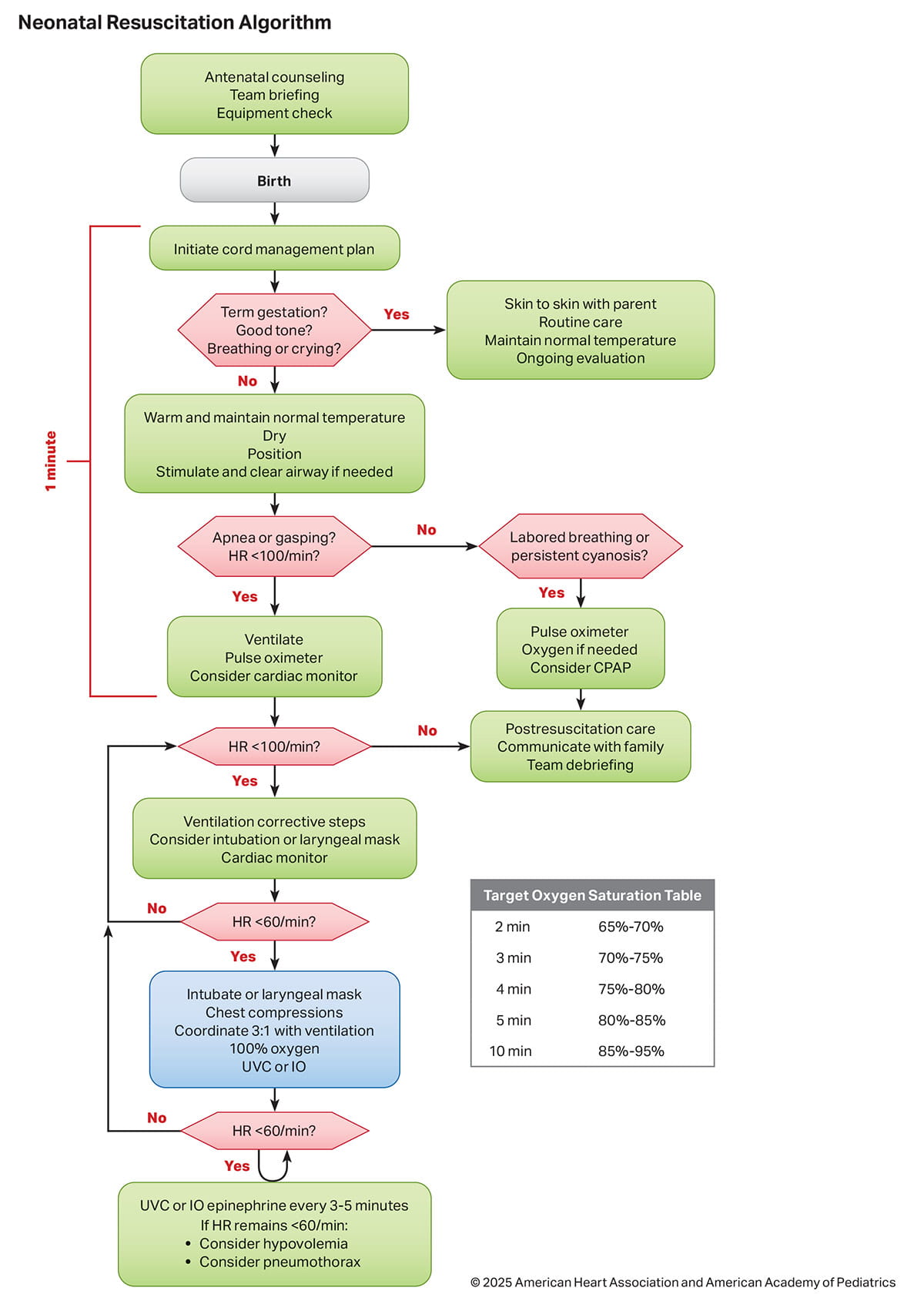
The 2025 Neonatal Resuscitation Algorithm summarizes the sequence of assessments and actions contained in the guidelines (1Figure 2).
Neonatal resuscitation science continues to advance. Although major updates to the guidelines occur every 5 years, interim updates may occur in the form of focused updates, as further evidence emerges based on publications of new clinical trials and systematic reviews.16 Gaps in knowledge relating to neonatal resuscitation remain. Some recommendations are based on weak evidence with only a few well-designed human studies. Therefore, the guidelines conclude with a summary of current gaps in neonatal resuscitation research.
These guidelines apply primarily to the newborn infant who is transitioning from a fluid-filled to an air-filled environment. The concepts in these guidelines may also apply to infants during the neonatal period (birth to 28 days of age) and potentially older infants cared for in the neonatal intensive care unit, depending on patient pathophysiology and institutional practice.17 Institutional policies and practices should be established based on the predominant patient population profile (in terms of age and pathophysiology) and staff training. Resuscitation of infants and children is addressed in “Part 6: Pediatric Basic Life Support” and “Part 8: Pediatric Advanced Life Support.”18,19
Evidence Evaluation and Guidelines Development
The following sections briefly describe the process of evidence review and guideline development. Refer to “Part 2: Evidence Evaluation and Guidelines Development” for more details on this process.20
Organization of the NLS Writing Group
The Neonatal Life Support Writing Group includes physicians, nurses, and scientists with backgrounds in neonatology, obstetrics, education, research, and public health. A call for candidates was distributed to the American Heart Association (AHA) Emergency Cardiovascular Care (ECC) Committee and American Academy of Pediatrics (AAP) subject matter experts, and volunteers with recognized expertise in neonatal resuscitation were nominated by the writing group co-chairs. Writing group members were selected by the AHA ECC Science Subcommittee and the AAP Executive Committee and then approved by the AHA Manuscript Oversight Committee. The AHA and AAP have rigorous conflict of interest policies and procedures to minimize the risk of bias or improper influence during development of the guidelines.21 Before appointment, writing group members and peer reviewers disclosed all commercial relationships and other potential (including intellectual) conflicts. Comprehensive disclosure information for writing group members is listed in Appendix 1. Writing group members whose research led to changes in guidelines were required to declare those conflicts during discussions and abstain from voting on those specific recommendations. This process is described more fully in “Part 2: Evidence Evaluation and Guidelines Development.”20
Methodology and Evidence Review
These 2025 AHA and AAP neonatal resuscitation guidelines are based on the extensive evidence evaluation performed in conjunction with the ILCOR and affiliated ILCOR member councils. Three different types of evidence reviews (systematic reviews, scoping reviews, and evidence updates) were used in the 2025 process. Each of these resulted in a description of the literature that facilitated guideline development.22 This process is described more fully in “Part 2: Evidence Evaluation and Guidelines Development.”20
Class of Recommendation and Level of Evidence
The writing group reviewed all relevant and current AHA guidelines for cardiopulmonary resuscitation and ECC16,23 and all relevant ILCOR consensus on science with treatment recommendations evidence and recommendations to determine if current guidelines should be reaffirmed, revised, or retired, or if new recommendations were needed.24-28 Supplemental reviews were performed by the writing groups to develop recommendations. The writing groups then drafted, reviewed, and approved recommendations, assigning to each a Level of Evidence (LOE; ie, quality) and Class of Recommendation (COR; ie, strength) (Table 1). Refer to “Part 2: Evidence Evaluation and Guidelines Development.”20
Open table in a new window.
Guideline Structure
The 2025 guidelines are organized into knowledge chunks, discrete modules of information on specific topics or management issues.30 Each modular knowledge chunk includes a table of recommendations using standard AHA nomenclature of COR and LOE. A short synopsis is provided to put the recommendations into context with important background information and overarching management or treatment concepts. Recommendation-specific text clarifies the rationale and key study data supporting the recommendations. Hyperlinked references are provided to facilitate quick access and review.
Document Review and Approval
Each 2025 Guidelines document was given guidance and reviewed by the AHA’s ECC Science Subcommittee leadership and submitted for blinded peer review to 16 subject matter experts nominated by the AHA and the AAP, when appropriate. Before appointment, all peer reviewers were required to disclose relationships with industry and any other potential conflicts of interest, and all disclosures were reviewed by AHA staff. Peer reviewer feedback was provided for guidelines in draft format and again in final format. All guidelines were reviewed and approved for publication by the AHA Science Advisory and Coordinating Committee, the AAP Board of Directors, and the AHA Executive Committee. Comprehensive disclosure information for peer reviewers is listed in Appendix 2. These recommendations supersede the last full set of AHA recommendations for neonatal life support, made in 2020.23 The writing group members voted on each individual recommendation and approved all guideline recommendations.
Major Concepts
The primary goal of neonatal care at birth is to facilitate transition of the fetus from the fluid-filled uterine environment and reliance on placental circulation to the newborn infant receiving oxygenation and air exchange through the lungs. The most important priority for newborn survival is the establishment of adequate lung inflation and ventilation after birth. Consequently, all births should be attended to by at least 1 person skilled and equipped to provide ventilation. Other important goals include establishment and maintenance of cardiovascular and temperature stability, as well as the promotion of parent-infant bonding and breastfeeding, recognizing that simple measures can avert disruption of transition in healthy newborn infants.
The Neonatal Resuscitation Algorithm has been updated since 2020 and is the organizing framework for major concepts that support the needs of the newborn infant, the family, and the surrounding team of perinatal caregivers (Figure 2).
Anticipation and Preparation for Resuscitation
Every newborn infant should have a trained and equipped person, or team when appropriate, to facilitate transition after birth. Identification of risk factors may indicate the need for additional personnel and equipment. Team behaviors such as anticipation, communication, briefing, equipment checks, and role assignment result in improved performance and outcomes.
Umbilical Cord Management
The neonatal team can communicate and plan with the obstetric team to provide the most appropriate cord management, which in most cases will be deferring cord clamping for at least 60 seconds. This applies to both preterm and term newborn infants who do not require resuscitation.
Initial Steps
When possible, term newborn infants should be managed skin-to-skin with their parent. After birth, the newborn infant with cord intact can be placed directly skin-to-skin, with attention to warm coverings and maintenance of normal temperature, while receiving ongoing evaluation of respiratory transition. Radiant warmers and other warming adjuncts are suggested for newborn infants who require resuscitation at birth, especially those born very preterm.
Stimulation may be provided to facilitate respiratory effort. Suctioning may be considered for suspected airway obstruction but is not routinely recommended.
Assessment of Heart Rate
Heart rate is assessed initially by auscultation. Pulse oximetry and electrocardiography (ECG) are important adjuncts in babies requiring resuscitation.
Ventilation and Continuous Positive Airway Pressure
Ventilation, also referred to as positive pressure ventilation, remains the primary method for providing support for newborn infants who are apneic, bradycardic, or demonstrate inadequate respiratory effort. Most newborn infants will respond to this intervention. An improvement in heart rate and establishment of breathing or crying are all signs of effective ventilation, which may be aided by corrective steps. The laryngeal mask, also known as a supraglottic airway, can be an effective method for providing ventilation to the newborn infant. For preterm infants who are breathing spontaneously but having increased work of breathing or hypoxia, CPAP may be beneficial.
Oxygen Supplementation
Ventilation may be initiated with air (21% oxygen) in term and late preterm newborn infants, and 21% to 30% oxygen in preterm babies 32 to 35 weeks of gestation. Very preterm infants less than 32 weeks’ gestational age may require higher oxygen concentrations (30%–100%) to achieve target oxygen saturation goals guided by pulse oximetry.
Chest Compressions
If the heart rate remains less than 60/min despite 30 seconds of ventilation that moves the chest, preferably through an alternative airway (endotracheal tube or laryngeal mask), chest compressions should be provided. The suggested ratio is 3 chest compressions synchronized to 1 inflation (with 30 inflations per minute and 90 compressions per minute). The preferred method is the 2 thumb–encircling hands technique, not the 2-finger or other techniques.
Intravascular Access
When intravascular access is required in the newborn infant, the umbilical venous route is preferred. When intravenous access is not possible or feasible, the IO route may be considered.
Epinephrine
If the heart rate remains less than 60/min despite 60 seconds of chest compressions and adequate ventilation, epinephrine should be administered, ideally via an intravascular route. Endotracheal epinephrine may be considered while vascular access is being obtained.
Volume Expansion
When blood loss is known or suspected based on history and examination, and there is no response to intravascular epinephrine, volume expansion is indicated.
Postresuscitation Care
If prolonged ventilation or advanced resuscitation is required, newborn infants should be closely monitored after stabilization. This monitoring may identify complications that can impact short- and long-term outcomes.
Withholding and Discontinuing Resuscitation
It may be possible to identify conditions in which withholding or discontinuing resuscitative efforts may be reasonably considered by families and health care professionals. Appropriate and timely support for collaborative decision-making should be provided to all involved.
Training and Human Performance
To optimize performance, teams and individuals who provide neonatal resuscitation should acquire necessary knowledge, technical skills, and behaviors, and also ensure ongoing maintenance of these skills. Given the low frequency of advanced resuscitation and inconsistency of resuscitation team members, institutions should be intentional in creating opportunities for individual and team training. Such training should include ongoing booster training and the use of simulation and debriefing.
Terminology
Prior neonatal guidelines have used the term positive pressure ventilation to refer to providing assisting ventilation. In this update, we generally use the term ventilation or assisted ventilation in order to simplify terminology, to align with other guidelines, and with the recognition that ventilation in neonatal resuscitation only involves positive pressure, not negative pressure.
The broad categories of gestational age can be specified as term to encompass infants 37+0 to 41+6/7 weeks’ gestational age, while preterm describes infants born prior to 37 weeks’ gestational age. When term and preterm are used in isolation in the guidelines, the above categorization would apply. When the evidence applies to a more specific subgroup, those gestational ages are specified in the recommendation and supportive text.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Every birth should be attended by at least 1 person whose only responsibility is the care of the newborn infant, including performing the initial steps of resuscitation and providing ventilation when required. |
| 1 | B-NR | 2. Before every birth, a standardized risk factors assessment should be performed to determine perinatal risk and assemble a neonatal resuscitation team based on that risk. |
| 1 | C-LD | 3. Before every birth, a standardized checklist should be used to ensure the presence and function of supplies and equipment necessary for a complete resuscitation. |
| 1 | C-LD | 4. When anticipating a high-risk birth, a prebirth team briefing should be performed to identify potential interventions and assign roles and responsibilities. |
Synopsis
Approximately 5% to 10% of newborns require assistance to breathe after birth.1-3 Newborn resuscitation requires training, preparation, and teamwork. Delays in assisting an apneic newborn may increase risk of death.2,4,5 Therefore, it is optimal that every birth has at least 1 person in attendance whose primary responsibility is the newborn infant and who can provide ventilation.6-8
A risk assessment to evaluate factors during pregnancy and labor can identify newborn infants likely to require resuscitation; in these cases, a team with appropriate skills should be present at birth.4,9-15 In the absence of risk stratification, up to half of babies requiring ventilation may not be identified before birth.2,16
A standardized checklist is a comprehensive list of critical supplies and equipment needed in the clinical setting. A standardized checklist used before every birth can ensure that supplies and equipment for a complete resuscitation are present and functional.17,18
A prebirth team briefing can identify the leader, assign roles and responsibilities, and plan umbilical cord management and potential interventions. Team briefings promote teamwork, communication, and safety but may delay communication with families.17,19-22 Facilitating communication with family during resuscitation can be considered in role assignment.21,22
Recommendation-Specific Supportive Text
- A large observational study found that delaying ventilation increases risk of death and prolonged hospitalization.5 A systematic review and meta-analysis of pre-post training studies showed neonatal resuscitation training improved 7-day neonatal survival in low-resource countries.8 A retrospective cohort study showed improved Apgar scores in the context of a statewide neonatal resuscitation training program.23 As the need for neonatal resuscitation can be unexpected, having a dedicated and trained clinician is optimal.
- Risk factors for low Apgar scores and receipt of assisted ventilation or advanced resuscitation procedures include prematurity, maternal conditions, delivery mode, and meconium-stained fluid.9-15 In a prospective survey done before risk stratification, resuscitation was anticipated in less than half of births receiving ventilation.16 In a prospective cohort study, risk stratification increased attendance of an advanced neonatal resuscitation team at high-risk births.4
- Checklist use led to reduced error rates during audits in neonatal resuscitation equipment and supplies. Quality improvement and implementation work that incorporated checklist use was associated with quicker time to oxygen saturation monitoring, reduced hypothermia for preterm infants, and increased deferred cord clamping rates.21,22,24
- Quality improvement initiatives have demonstrated that team briefing including role assignment, in combination with checklists, improved team communication and was associated with quicker time to oxygen saturation monitoring and normothermia for preterm infants.21,22,24
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. For term newborn infants who do not require immediate resuscitation, deferred cord clamping for at least 60 seconds can be beneficial when compared to immediate cord clamping. |
| 2b | B-R | 2. For nonvigorous term newborn infants and late preterm infants 35 weeks or more gestational age, intact cord milking may be reasonable when compared to immediate cord clamping. |
| 2b | C-LD | 3. For term newborn infants who do not require immediate resuscitation, the usefulness of intact umbilical cord milking compared to deferred cord clamping is uncertain. |
Synopsis
Management of the umbilical cord and placental transfusion at the time of birth remains an area of robust investigation. Placental transfusion can be achieved either by delaying the clamping of the umbilical cord following delivery or by milking the umbilical cord. Milking is typically done by stripping 20 cm of cord from the placenta toward the infant 3 to 4 times, allowing the cord to refill from the placenta each time. The volume of blood transferred from the placenta to the newborn infant and the impact of this transfusion vary based on gestational age at birth, mode of delivery, the time from birth to cord clamping, any milking of the umbilical cord, and physiologic status of the newborn. Based on available literature, deferred cord clamping for at least 60 seconds can be beneficial compared to immediate cord clamping for term infants who do not require resuscitation. For term infants who require resuscitation at birth, there is limited evidence to guide timing of umbilical cord clamping. Several studies currently are underway to investigate the interaction between deferred cord clamping and resuscitation. Initial steps of resuscitation such as drying and tactile stimulation, if required, can be undertaken during deferred cord clamping.
Recommendation-Specific Supportive Text
- For term newborns, evidence from 14 studies (n=2412)1-14 reporting on hemoglobin, and 20 studies (n=3452)1,4-6,8,10,13,15-27 reporting on hematocrit showed that infants received deferred umbilical cord clamping beyond 30 seconds had improved hematologic indices early in the newborn period. In 5 trials studying 1199 term newborn infants, those who received early cord clamping had lower ferritin levels at 3 to 6 months of age compared to those who received deferred cord clamping.1,6,18,28,29 There was no difference in mortality among term infants who received deferred or early cord clamping in 3 randomized controlled trials (RCTs) including 419 infants.16,18,30 More recent studies have evaluated deferred clamping for at least 60 seconds3,11-13,15,18,23,25,31-34 and additional studies have evaluated longer durations of deferred umbilical cord clamping.17,35-37 These studies have shown increases in hematological indices compared to shorter durations of deferred cord clamping.
- One RCT including 1730 nonvigorous infants, limited to 35 to 42 weeks’ gestational age, compared intact umbilical cord milking to early cord clamping (within 60 seconds of birth).38 The primary outcome, admission to neonatal intensive care unit, was not different between groups. However, compared to early cord clamping, intact umbilical cord milking in nonvigorous infants was associated with decreases in several secondary outcomes, including rate of cardiorespiratory support in the delivery room, moderate to severe hypoxic-ischemic encephalopathy (HIE), and use of therapeutic hypothermia.
- The currently available evidence does not support intact cord milking compared to deferred cord clamping in vigorous term newborns.31,32,39 Two RCTs including 275 term infants showed no difference between hematologic indices when measured between 6 and 48 hours after delivery.31,39 One study followed subjects to 4 months of age and measured hemoglobin and ferritin. No difference was noted between the cord milking and delayed cord clamping groups.31 Two RCTs including 371 term infants did not demonstrate a difference in hyperbilirubinemia, administration of phototherapy, or need for exchange transfusions.32,39
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. For newborn infants born at less than 37 weeks of gestation who do not require immediate resuscitation, deferred cord clamping for at least 60 seconds is recommended when compared to immediate cord clamping. |
| 2b | B-R | 2. For newborn infants born at 28+0 to 36+6 weeks of gestation who do not require immediate resuscitation and in whom deferred cord clamping cannot be performed, intact cord milking may be reasonable. |
| 3: Harm | B-R | 3. For newborn infants born at less than 28+0 weeks of gestation, intact cord milking should not be performed. |
Synopsis
Outcomes of interest and recommendations for cord management in preterm infants differ from term infants because of differences in the cardiovascular and cerebral vascular physiology of preterm infants. Transfer of blood from the placenta through the umbilical vein after birth has been shown to increase hemoglobin, increase circulating blood volume, improve organ perfusion and result in greater hemodynamic stability in preterm newborns after birth.40-42 Based on available evidence for preterm infants who do not require resuscitation, deferred cord clamping for at least 60 seconds may result in improved outcomes. While deferred cord clamping is the preferred approach, an alternative approach is intact cord milking for infants born at 28+0 to 36+6 weeks’ gestational age in whom deferred cord clamping cannot be performed.43 For infants born at less than 28 weeks’ gestational age, intact cord milking is associated with increased risk of intraventricular hemorrhage.43,44 Some studies have shown an increased incidence of hypothermia on admission to the neonatal intensive care unit for preterm infants who received deferred cord clamping when compared to those who received immediate cord clamping. Attention to maintaining normothermia is an important aspect of care during resuscitation regardless of cord management strategy.
Recommendation-Specific Supportive Text
- A recent pairwise individual participant data meta-analysis conducted by ILCOR included 21 studies enrolling 3292 newborns with a median gestational age of 29 weeks (IQR range 27–33 weeks) and compared deferred cord clamping (30 to ≥180 seconds) to immediate cord clamping (≤15 seconds).22,43,45-62 Twenty trials enrolling 3263 infants showed a reduction in mortality before discharge with a number needed to treat of 40 (95% lCI 143 to 26 infants).22,45-63 For all preterm infants, there is an increase in hematological indices,43 while in those infants born at less than 32 weeks’ gestational age, there is a reduction in need for packed red blood cell transfusion during neonatal intensive care unit admission.22,46,48-50,52,53,55,57,59,61-63 In comparing durations of deferred cord clamping, long duration (≥120 seconds) was associated with the greatest reduction in mortality, but the number of extreme preterm infants included in these studies was low (n=121) and adherence to this duration (67%) was significantly lower compared to adherence for short and medium durations (30 seconds to less than120 seconds).43 A post-hoc analysis by ILCOR demonstrated that newborn infants who received deferred cord clamping for 60 seconds or more (n=1316) had a reduction in mortality compared to those who had immediate cord clamping (less than15 seconds) (odds ratio [OR], 0.63 [95% lCI 0.44–0.88, p=0.01]).1543,64 In 8 trials including 1995 preterm infants, hypothermia (defined as less than 36.5 °c) was increased on admission to the neonatal intensive care unit for infants less than 32 weeks of gestation who received deferred cord clamping compared to immediate cord clamping (mean difference -0.13 degrees, OR 1.28).43
- In preterm infants born at ≥28 weeks’ gestational age who received intact umbilical cord milking, pairwise individual participant data meta-analysis of 12 trials including 944 infants showed that hemoglobin levels in the first 24 hours after birth were higher compared with those who received immediate cord clamping.43 Further, data from 15 studies including 1163 infants showed a reduction in the need for red blood cell transfusion for those infants who received intact cord milking compared with immediate cord clamping.43 For infants who received intact umbilical cord milking compared with deferred cord clamping, data from 7 studies including 860 infants showed an increased risk of severe intraventricular hemorrhage in infants born at less than 32 weeks’ gestational age. This was primarily related to 1 RCT including 540 infants which showed an increased incidence of intraventricular hemorrhage in infants born at less than 28 weeks’ gestational age.44 One RCT including 1019 infants delivered at 28 to 32 weeks of gestation showed no increased rate of severe intraventricular hemorrhage in the group receiving intact umbilical cord milking when compared with those receiving deferred cord clamping.65
- In a single study including 182 infants born between 23 and 27+6/7 weeks of gestation who did not require immediate resuscitation, the incidence of severe intraventricular hemorrhage was significantly higher in those who received umbilical cord milking compared with deferred cord clamping of 60 seconds.44
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. The temperature of newborn infants should be monitored and maintained between 36.5 °C and 37.5 °C after birth through admission and stabilization. |
| 1 | B-NR | 2. Hypothermia (temperature less than 36 °C) should be prevented due to its association with adverse outcomes in newborn infants. |
| 1 | B-NR | 3. Hyperthermia (temperature greater than 38 °C) should be prevented due to its association with adverse outcomes in newborn infants. |
Synopsis
Temperature after birth is an important measure of resuscitation quality.1,2 The ideal temperature of newborn infants is between 36.5 °C and 37.5 °C.3 Hypothermia is associated with increased neonatal mortality and morbidity, especially in very preterm (less than 33 weeks’ gestational age) and very low-birth-weight infants (less than 1500 g), who are at increased risk for hypothermia.4-7 Hyperthermia is associated with increased mortality and cerebral injury in both term and preterm infants.5,8-10 Adverse outcomes are more frequent as temperature deviation on either side of the normal range increases.4,5
Recommendation-Specific Supportive Text
- There are long-standing worldwide recommendations for routine temperature management for the newborn infant.3 Monitoring of temperature after birth shows that hypothermia is common worldwide, with a higher incidence in newborn infants of lower gestational age and birth weight.4-7,11 Hyperthermia may occur when warming interventions are provided in the delivery room without careful monitoring.12
- In observational studies, the presence and degree of hypothermia after birth is strongly associated with increased neonatal mortality and morbidity, particularly in preterm (less than 37 weeks’ gestational age) and low-birth-weight infants (less than 2500 g).4-7
- Two observational studies found an association between hyperthermia and increased morbidity and mortality in very preterm and very low-birth-weight newborn infants.5,10 Two observational studies showed an association between hyperthermia in preterm infants and major abnormalities on cranial ultrasound, neurodevelopmental impairment, and necrotizing enterocolitis.8,13 Meta-analysis of 31 observational studies of epidural analgesia showed an association between intrapartum hyperthermia and adverse neonatal neurological outcome in both term and preterm infants.9
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. For preterm infants in the delivery room, the use of radiant warmers, plastic bags or wraps (with a cap), increased room temperature, and warmed humidified inspired gases, separately or in combination, can be effective in preventing hypothermia. |
| 2a | C-LD | 2. Placing newborn infants skin-to-skin after birth can be effective to maintain normothermia. |
| 2a | C-LD | 3. For newborn infants who require resuscitation, temperature-controlling interventions can be beneficial. |
| 2b | B-R | 4. For preterm infants, exothermic mattresses may be considered for the prevention of hypothermia. |
| 2b | B-NR | 5. For preterm infants, various combinations of warming strategies (or “bundles”) may be reasonable to prevent hypothermia but may also increase the risk of hyperthermia. |
Synopsis
Placing healthy newborn infants skin-to-skin after birth can be beneficial for maintaining normothermia.14 For preterm and low-birth-weight infants or newborn infants requiring resuscitation, warming adjuncts (ambient temperature greater than 23 °C, skin-to-skin care, radiant warmers, plastic wraps or bags, hats, blankets, exothermic mattresses, and warmed humidified inspired gases)15-17 individually or in combination may reduce the risk of hypothermia. However, exothermic mattresses have been reported to cause local heat injury and hyperthermia.18 Warming devices, especially when used in combination with other interventions to minimize heat loss, can result in hyperthermia.
Recommendation-Specific Supportive Text
- RCTs and observational studies of warming adjuncts, alone and in combination, demonstrate reduced rates of hypothermia in very preterm and very low-birth-weight infants.15,16,19 However, meta-analysis of RCTs of interventions that reduce hypothermia in very preterm or very low-birth-weight infants show no impact on neonatal morbidity or mortality.16 Two RCTs and expert opinion support ambient temperatures of 23 °C and above.3,17,20
- Two meta-analyses of RCTs showed that early skin-to-skin contact promotes normothermia in healthy newborn infants.2,14 A single-center RCT comparing immediate skin-to-skin contact during and after elective cesarean delivery with conventional management showed elimination of hypothermia and hypoglycemia in the intervention group, as well as improved breastfeeding outcomes.21 Two meta-analyses reviewed RCTs and observational studies of extended skin-to-skin care after initial resuscitation or stabilization, some in resource-limited settings, showing reduced mortality, improved breastfeeding and blood glucose stability, shortened length of stay, and improved weight gain in preterm and low-birth-weight infants.22,23
- Most RCTs in well-resourced settings routinely manage at-risk babies and those requiring resuscitation under a radiant warmer.15,16 Other warming adjuncts are utilized when resuscitation is initiated with the cord intact.12
- Several RCTs suggest that exothermic mattresses (combined with other measures) prevent hypothermia in preterm infants.19 One RCT found higher rates of hyperthermia with exothermic mattresses.18
- Numerous nonrandomized quality improvement studies support the combined use of multiple warming adjuncts (“bundles”).24 The use of radiant warmers in manual mode or exothermic mattresses in combination with other interventions to conserve heat effectively maintains temperature but increases the risk of hyperthermia.19
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. Tactile stimulation can be useful in newborn infants who appear to have ineffective respiratory effort after birth. |
Synopsis
The immediate care of newborn infants involves an initial assessment of breathing and muscle tone. Infants who are breathing well or crying are cared for skin-to-skin with their mothers and do not need interventions such as routine tactile stimulation.25 Tactile stimulation is defined as drying an infant and rubbing the back and soles of the feet.25 If there is ineffective breathing effort or apnea after birth, tactile stimulation may improve or initiate breathing. There may be some benefit from repeated tactile stimulation in preterm infants during or after providing ventilation, but this requires further study.26 Prolonged tactile stimulation should not delay the provision of ventilation.
Recommendation-Specific Supportive Text
- Limited observational studies suggest that tactile stimulation may improve respiratory effort. In an observational study of video recordings of neonatal resuscitations among 245 infants born prior to 32 weeks of gestation, those who received tactile stimulation had lower rates of endotracheal intubation than those who did not receive stimulation.27 One RCT found improved oxygenation after resuscitation in preterm infants who received repeated tactile stimulation.26 This study concerned only preterm infants 27 to 32 weeks’ gestational age. In an observational study of 3073 noncrying newborn infants 34 weeks or more of gestation, 83% received stimulation, of whom 81% breathed after stimulation when cord was intact, while 69% breathed after stimulation with cord clamped.28
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO | 1. Suctioning of the mouth and nose can be considered in newborn infants if ventilation is required and the airway appears obstructed. |
| 2a | C-EO | 2. Intubation and tracheal suction can be beneficial for newborn infants who have evidence of tracheal obstruction during ventilation. |
| 3: No Benefit | B-R | 3. Routine oral, nasal, oropharyngeal, or endotracheal suctioning of newborn infants is not recommended, regardless of whether the fluid is clear or meconium-stained. |
Synopsis
Newborn infants who are breathing well or crying are cared for skin-to-skin with their mothers and should not need interventions such as suctioning, whether the amniotic fluid is clear or meconium-stained.29 Avoiding unnecessary suctioning helps prevent the risk of induced bradycardia, apnea, desaturation, and airway injury.29 If, at initial assessment, there is visible fluid obstructing the airway or a concern about obstructed breathing, the mouth and nose may be suctioned. Suctioning is reserved for when there is evidence of airway obstruction during ventilation. Direct laryngoscopy and endotracheal suctioning had been practiced in the past for newborn infants with meconium-stained amniotic fluid. However, this practice has not been shown as beneficial, whether the infant is vigorous or nonvigorous.30 The practice of suctioning may delay assisted ventilation. When ventilation appears ineffective after corrective measures, airway obstruction may be present from blood, mucous, amniotic fluid, or meconium. In those instances, providing suction to relieve the obstruction may facilitate more effective ventilation.
Recommendation-Specific Supportive Text
- Suctioning for suspected airway obstruction during ventilation is based on expert opinion. When ventilation is indicated and the airway appears obstructed, use of a bulb syringe or other suction device can clear the airway. There is no evidence or consensus to support the use of deep suctioning of the stomach.
- Endotracheal suctioning for apparent airway obstruction is based on expert opinion. Regardless of the presence of meconium, when ventilation corrective steps do not lead to an increase in heart rate or to chest rise, endotracheal suctioning may help to relieve an obstruction. Obstruction may be caused by meconium, mucous plug, blood, or amniotic fluid.
- A meta-analysis of 7 RCTs found no benefit from routine upper airway suctioning after birth in infants born through clear amniotic fluid.29 Two RCTs and 2 prospective observational studies found lower oxygen saturations in infants who received suctioning within the first 10 minutes, while 2 RCTs did not find significant differences.29 A meta-analysis of 4 RCTs found no benefit of endotracheal suction for infants born through meconium-stained amniotic fluid.30 A large quality improvement trial found that avoiding suction prior to spontaneous breathing, but only wiping the mouth of nonvigorous infants with a towel, was associated with decreased exposure to oxygen in the delivery room (12.4% versus 4.4%) but no differences in Apgar scores, use of ventilation or CPAP, or neonatal intensive care unit admission.31
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. ECG can be useful for fast and accurate heart rate assessment in newborn infants. |
| 2a | C-LD | 2. Auscultation and pulse oximetry are reasonable alternatives to ECG for heart rate assessment in newborn infants. |
Synopsis
After birth, the newborn infant’s heart rate is used to assess the effectiveness of spontaneous respiratory effort, the need for interventions, and the response to interventions. Auscultation of the precordium remains the preferred physical examination method for the initial assessment of the heart rate, although assessment by auscultation (or palpation) may be unreliable and inaccurate. ECG provides the most rapid and accurate measurement of the newborn infant’s heart rate at birth and during resuscitation.1 Compared to ECG, pulse oximetry is both slower in detecting the heart rate and tends to be inaccurate during the first few minutes after birth. Several emerging technologies show potential for fast and accurate heart rate assessment, but insufficient clinical data exists for inclusion in these recommendations. Underestimation of heart rate can lead to potentially unnecessary interventions. Alternatively, overestimation of heart rate when a newborn infant is bradycardic may delay necessary interventions. Limited data are available comparing the different approaches to heart rate assessment during neonatal resuscitation on other neonatal outcomes. Use of ECG for heart rate detection does not replace the need for pulse oximetry to evaluate oxygen saturation to assess the need for supplemental oxygen. Pulse oximetry can be placed in anticipation of resuscitation or when assisted ventilation is initiated. ECG can be considered when assisted ventilation is initiated and placed by the time that an alternative airway is being considered for bradycardia (Figure 2).
Recommendation-Specific Supportive Text
- In 1 RCT and 1 observational study including 70 infants, there were no reports of technical difficulties with ECG monitoring during neonatal resuscitation, supporting its feasibility as a tool for monitoring heart rate during neonatal resuscitation.2,3 One observational study including 630 infants compared neonatal outcomes before (historical cohort) and after implementation of ECG monitoring in the delivery room.4 Compared with the newborn infants in the historical cohort, newborn infants with ECG monitoring had lower rates of endotracheal intubation and higher 5-minute Apgar scores. Newborn infants in that study with ECG monitoring had higher odds of receiving chest compressions in the delivery room with appropriateness of compressions not assessed. Evidence from 11 nonrandomized studies2,5-14 enrolling 452 newborn infants and 3 RCTs15-17 enrolling 187 newborn infants suggest that at birth, ECG is faster and more accurate for newborn heart assessment compared with pulse oximetry. Data from 4 nonrandomized studies enrolling 156 newborns and 1 RCT enrolling 45 newborn infants show that auscultation is not as accurate as ECG for heart rate assessment during stabilization immediately after birth.6,18-21
- In the absence of ECG, studies demonstrate auscultation and pulse oximetry are faster or more accurate than palpation for heart rate assessment.1,18 When ECG is unavailable or not functional, auscultation or pulse oximetry are reasonable alternatives and adjuncts for heart rate assessment.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. ECG should be used for the rapid and accurate assessment of heart rate during chest compressions in term and preterm infants. |
Synopsis
When chest compressions are initiated, ECG monitoring can confirm heart rate. The placement of ECG leads can facilitate a continuous assessment of heart rate without the need for a team member auscultating the heart intermittently. During chest compressions, the ECG signal may not be reliable due to artifact. When ECG heart rate is greater than 60/min, a palpable pulse or audible heart rate rules out pulseless electrical activity.22-26
Recommendation-Specific Supportive Text
- Given its reliability in heart rate assessment compared to other modalities prior to beginning chest compressions in term and preterm infants, expert opinion is that ECG should be used when providing chest compressions despite the lack of direct evidence supporting the practice during chest compressions.1
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Ventilation should be provided within 60 seconds after birth in newborn infants who are gasping or apneic or who are persistently bradycardic (heart rate less than 100/min) despite appropriate initial steps. |
| 2a | C-LD | 2. For newborn infants, initial peak inflation pressures of 20 to 30 cm H2O are reasonable, with adjustment of peak inflation pressures to provide effective ventilation. |
| 2b | C-LD | 3. In newborn infants receiving ventilation, it may be reasonable to provide positive end-expiratory pressure (PEEP). |
| 3: Harm | C-LD | 4. Excessive peak inflation pressure can result in high tidal volume, which is potentially harmful and should be avoided. |
Synopsis
Most newborn infants start breathing spontaneously within 30 to 60 seconds after birth, sometimes with the help of drying and gentle stimulation.1 If a newborn infant does not breathe spontaneously or has a persistent heart rate below 100/min despite initial efforts including tactile stimulation, ventilation is required at a rate of 30 to 60 inflations per minute. Delay in providing ventilation can increase the risk of death.1 The effectiveness of ventilation is assessed by increase in heart rate and, to a lesser extent, by chest movement. For newborn infants, initial peak inflation pressures of 20 to 30 cm H2O are generally sufficient to inflate the lungs. In some cases, higher peak inflation pressures may be necessary.2,3 However, excessive tidal volumes have been associated with lung and brain injury in preterm newborns.4,5 As tidal volumes can be both insufficient for effective ventilation if too low, or injurious if too high, titration of peak inflation pressure can be considered during neonatal resuscitation.
Lungs of sick or preterm infants are prone to collapse due to immaturity or inflammation. PEEP improves lung function and oxygenation, and maintains lung inflation during expiration. While PEEP may be advantageous, human studies in neonates are limited, and the optimal level of PEEP remains undetermined.6,7
Recommendation-Specific Supportive Text
- Most nonvigorous newborn infants respond to stimulation and ventilation. The risk of death or prolonged hospitalization increases by 16% for every 30-second delay in initiating ventilation.1
- An observational study in 129 term newborn infants using a T-piece resuscitator (refer to the Devices for Ventilation section) reported that during ventilation, median (iIQR) peak inflation pressures of 30.6 (28.6–31.6) cm H2O resulted in a tidal volume of 4.5 (1.6–7.8) mL/kg.2 Similarly, an observational study in 821 term newborn infants using a self-inflating bag reported that during ventilation, median peak inflation pressure of 37.7 (32.6–40.8) cm H2O resulted in a tidal volume of 5.2 (2.3–8.6) mL/kg.3 Furthermore, a multicenter cluster-crossover trial randomized preterm infants greater than 26 weeks’ gestational age and term infants to ventilation with either a self-inflating bag with or without a PEEP valve or a T-piece with PEEP and reported a mean (standard deviation) maximum peak inflation pressure of 26 (2) cm H2O with the T-piece versus 28 (5) cm H2O with the self-inflating bag.8 Observational studies in preterm newborn infants reported that a peak inflation pressure of 20 to 25 cm H2O delivered a median (IQR) tidal volume of 9 (3–15.7) mL/kg during ventilation, but higher initial pressures may sometimes be required.9-14 After initiation of assisted ventilation, titrating peak inflation pressures both up and down during resuscitation may be needed to achieve effective, but not excessive ventilation.
- A cohort study of 1962 infants born between 23 and 33 weeks’ gestational age reported lower rates of mortality and chronic lung disease during ventilation with PEEP versus ventilation with no PEEP.6 Furthermore, using logistic regression with adjustment for maternal and neonatal characteristics, use of the T-piece resuscitator with PEEP compared with self-inflating bag without PEEP increased the chance of survival to hospital discharge without major morbidities (OR, 1.38; 95% CI, 1.06–1.80).6
In preterm animal models, PEEP facilitates lung aeration and development of functional residual capacity, prevents distal airway collapse, increases lung surface area and compliance, decreases expiratory resistance, conserves surfactant, and reduces hyaline membrane formation, alveolar collapse, and the expression of proinflammatory mediators.15-17 - Animal studies reported that ventilation with high volumes initiates inflammatory cascades, which result in lung and brain injury in immature animals.18,19 The 2 pathways (cerebral blood flow instability and cerebral inflammatory cascade) increase the risk of brain injury and potential life-long adverse neurodevelopmental outcomes.18
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. It is reasonable to provide ventilation at a rate of 30 to 60 inflations per minute in newborn infants. |
| 2a | C-LD | 2. It is reasonable to initiate ventilation with an inflation time of 0.5 to 1 seconds in newborn infants. |
| 3: Harm | B-R | 3. In preterm newborn infants less than 28 weeks of gestation, the routine use of sustained inflations (>5 seconds) to initiate ventilation is potentially harmful and should not be performed. |
Synopsis
During ventilation, an inflation time of 0.5 to 1 second during ventilation aligns with the natural breathing patterns of both term and preterm newborn infants.2,20,21 It is appropriate to provide ventilation at a rate of 30 to 60 inflations per minute for newborn infants who are not breathing effectively.1,2 Studies that explored the effects of sustained inflations greater than 5 seconds reported higher risks for preterm newborns.22 The potential benefits or risks of sustained inflations lasting between 1 and 5 seconds remain unclear.
Recommendation-Specific Supportive Text
- An observational study of 129 term newborn infants reported that ventilation using inflation time of approximately 0.5 seconds with a rate 30 to 60/min resulted in delivered tidal volumes between 5 and 10 mL/kg.2 An observational study of 434 late preterm and term newborns reported that an inflation rate of 30/min was associated with the highest carbon dioxide clearance and the shortest time to reach exhaled carbon dioxide greater than 2%.23
- Observational studies including 950 term newborn infants reported that median inflation time of 0.46 seconds (IQR, 0.35–0.59 seconds) was associated with adequate tidal volume delivery.1,2 Furthermore, increased inflation times were associated with a rise in tidal volume.2
- An ILCOR task force review reported no benefit in using 1 or more sustained inflations of >5 seconds for preterm infants at birth.22 Sustained inflations may increase mortality among the subgroup of preterm newborns less than 28 weeks of gestation.22 The Sustained Aeration for Infant Lungs trial included 426 newborn infants 23 to 26 weeks’ gestational age and compared ventilation with up to 2 initial sustained inflations using a peak inflation pressure of 25 cm H2O for 15 seconds (intervention) to standard ventilation (control) and reported significantly higher early mortality with sustained inflation compared to standard ventilation.24
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1.Ventilation corrective maneuvers can be useful when initial positive pressure inflations are not effective for newborn infants who require ventilation. |
| 2a | B-R | 2. Video laryngoscopy can be useful for newborn infants who require endotracheal intubation. |
Synopsis
Effective ventilation is difficult to perform in newborn infants due to problems such as leak around the facemask, airway obstruction, and insufficient inflating pressures.25-28 Neonatal manikin studies have shown that large leaks around the facemask can occur during ventilation,29-32 and these findings have been replicated in clinical studies of term and preterm infants receiving mask ventilation.13,28,33-35 Ventilation corrective maneuvers to overcome common challenges to effective ventilation include airway repositioning, mask adjustment or use of the 2-handed mask hold, suctioning, increasing peak inflation pressure, and placement of an alternative airway.37
Direct laryngoscopy has been the traditional method of placing an endotracheal tube. As video laryngoscopes have been used both in the delivery room and in the neonatal intensive care unit, evidence has shown potential benefit including increased rates of first attempt success and overall success.38-43 As video laryngoscopy may not be feasible across all settings due to cost and training requirements, traditional laryngoscopy continues to be a reasonable alternative.
Recommendation-Specific Supportive Text
- In an infant manikin study of 48 subjects and a pilot RCT of 30 preterm infants, mask leak was significantly reduced when using a 2-handed mask hold technique compared to a single operator holding the mask and providing the ventilation.44,45 In an observational study of ventilation corrective maneuvers on the impact of tidal volume delivery during ventilation in 30 preterm infants, performing ventilation corrective steps led to improved tidal volume delivery in about one third of patients but resulted in ineffective or excessive tidal volumes in others. The number, sequence, and combination of maneuvers varied across resuscitations.46 In an observational study of physiological response to ventilation corrective maneuvers in 28 preterm infants, mask adjustment, airway repositioning, and increasing peak inflation pressure led to improved oxygen saturation. Mask adjustment and increasing peak inflation pressure were also observed to increase expired tidal volume, although excessive tidal volumes were seen in this study.47 An observational study of 58 term and 76 preterm infants reported a total of 427 maneuvers to improve noninvasive respiratory support. Cerebral regional tissue oxygen saturation measured by near infrared spectroscopy increased after the first ventilation corrective maneuver. There were nonsignificant improvements in heart rate, oxygen saturation, and facemask leak.48
- In a meta-analysis of 6 RCTs involving 862 intubations, video laryngoscopy compared to traditional laryngoscopy led to increased endotracheal intubation success.38-43,49 In a meta-analysis of 4 RCTs involving 610 intubations, video laryngoscopy compared to traditional laryngoscopy led to increased likelihood of endotracheal intubation on first attempt.39,41-43 In a meta-analysis of 4 RCTs involving 555 intubations, video laryngoscopy compared to traditional laryngoscopy led to decreased number of intubation attempts.38-40,43 Adverse outcomes such as airway trauma, bradycardia, oxygen desaturation, esophageal intubation, and pneumothorax were not increased in 5 comparative RCTs.39-43 In 4 observational studies involving 3342 intubations, video laryngoscopy compared to traditional laryngoscopy was associated with increased likelihood of endotracheal intubation on first attempt.50-53 While traditional laryngoscopy remains a reasonable method to achieve an alternative airway, video laryngoscopy may be preferable when resources and training allow.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. It is reasonable to use a laryngeal mask as an alternative to endotracheal intubation for newborn infants 34+0/7 weeks’ or more gestational age for whom ventilation via face mask is unsuccessful. |
| 2b | C-LD | 2. It may be reasonable to use a laryngeal mask as the primary interface to administer ventilation instead of a face mask for newborn infants 34+0/7 weeks’ or more gestational age. |
Synopsis
Available interfaces for delivery of ventilation during newborn resuscitation include face masks, nasal prongs, and laryngeal masks (also known as supraglottic airways). While face masks have historically been the most used and most studied interface in neonatal resuscitation, there is a growing body of evidence for the role of laryngeal masks as either primary or secondary interfaces for newborn ventilation.
Based on available evidence, these recommendations are limited to newborn infants greater than 34+0/7 weeks of gestation as this reflects the gestational age of the infants studied. Many manufacturers of laryngeal masks also state a lower weight limit of 2 kg, but smaller devices are now being made for infants weighing less than 2 kg. There is a lack of evidence for a lower weight limit or gestational age at which these smaller devices may be safely and effectively used.
Recommendation-Specific Supportive Text
- In 2 separate meta-analyses of the same 3 RCTs (158 infants born at greater than 34+0/7 weeks’ gestational age), there was no significant difference in insertion time, failure to correctly place the device, or first attempt success when a laryngeal mask was used as a secondary device instead of endotracheal intubation after face mask ventilation was unsuccessful.54,55 In 1 RCT (68 infants born at greater than 34+0/7 weeks’ gestational age) that was not included in these meta-analyses, there was no significant difference in insertion time, first attempt success, or duration of ventilation.56 In 1 observational study (86 infants born at 34+0/7 to 36+6/7 weeks’ gestational age) that was not included in these meta-analyses, use of a laryngeal mask instead of an endotracheal tube was associated with decreased likelihood of admission to the neonatal intensive care unit.57 There were insufficient subjects to determine statistical difference in important outcomes such as incidence of pneumothorax or mortality.
- A meta-analysis of 6 RCTs (1823 infants born at greater than 34+0/7 weeks’ gestational age) found that use of a laryngeal mask decreased the probability of failure to improve with the assigned device and the rate of endotracheal intubation in the delivery room when compared to use of a face mask.58 These studies had heterogeneity in regard to subjects in their resuscitation experience for face mask ventilation and tended to be conducted in low- and middle-income countries. The duration of ventilation and time until heart rate reached greater than 100/min were also shorter with the laryngeal mask. Similarly, a quasi-randomized study of 67 infants born at greater than 36 weeks’ gestational age that was not captured in the above meta-analysis found that infants who received ventilation using a laryngeal mask required shorter duration of ventilation and were less likely to require intubation when compared to infants who were ventilated using a face mask.59
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. It can be beneficial to use a T-piece resuscitator instead of a self-inflating bag, with or without a PEEP valve, for administering ventilation to newborn infants, particularly preterm infants. |
Synopsis
Several devices are available to administer ventilation, including self-inflating bags, flow-inflating bags, and T-piece resuscitators. The choice of ventilation device depends upon factors reflecting the context at a birthing site: the number of births, the case mix, availability of a compressed gas source, familiarity with the different devices, amount of training required to use each device, and device cost. Because both T-piece resuscitators and flow-inflating bags require a compressed gas source to function, a self-inflating bag can serve as a backup in the event of compressed gas failure when using either of these devices.
Recommendation-Specific Supportive Text
- A meta-analysis of 4 RCTs (1247 infants) found that resuscitation with a T-piece resuscitator compared with a self-inflating bag reduced the duration of assisted ventilation and decreased risk of bronchopulmonary dysplasia.7 A more recent meta-analysis of 5 RCTs (1271 infants) comparing “fixed pressure devices” (ie, T-piece resuscitators and mechanical ventilators) to “hand pressure devices” (ie, self-inflating bags and flow-inflating bags) similarly found that fixed pressure devices were associated with decreased bronchopulmonary dysplasia, intubation in the delivery room, requirement and duration of mechanical ventilation, and surfactant administration.60 Neither meta-analysis showed a difference in mortality between groups. Although subgroup analyses by gestational age were not feasible7 or not significant,60 bronchopulmonary dysplasia is an outcome that affects preterm infants and the use of a T-piece resuscitator may provide the greatest benefit to preterm infants. Since these meta-analyses, 2 additional RCTs (92 preterm infants) comparing the use of a self-inflating bag and T-piece resuscitator have been published. Ventilation provided by a T-piece resuscitator delivered tidal volumes within goal range more consistently when compared to a self-inflating bag,61 but there was no significant difference in duration of assisted ventilation or other outcomes.61,62 There were no new studies identified that evaluated the use of flow-inflating bags.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | A | 1. For spontaneously breathing preterm infants who require respiratory support immediately after birth, it is reasonable to use CPAP rather than intubation and mechanical ventilation. |
| 2b | C-LD | 2. For spontaneously breathing preterm infants who require respiratory support immediately after birth, the effectiveness of high-flow nasal cannula compared to CPAP is not well-established. |
| 2b | C-LD | 3. The usefulness of CPAP for spontaneously breathing term and late preterm infants 34 weeks’ or more gestational age who have or are at risk of having respiratory distress immediately after birth is not well-established. |
Synopsis
Newborn transition is dependent on lung inflation and establishing functional residual capacity.63 Most spontaneously breathing term and late-preterm newborn infants will achieve this goal independently. However, some infants, particularly preterm infants, will need assistance to establish functional residual capacity even while spontaneously breathing. These infants can have signs of respiratory distress (grunting, nasal flaring, retractions) or persistent hypoxia if they have not been independently successful in adequate pulmonary recruitment. CPAP can provide noninvasive support to infants to recruit alveoli for respiration and aid in transition.
Recommendation-Specific Supportive Text
- Four RCTs and 1 meta-analysis64-68 showed reduction in the combined outcome of death and bronchopulmonary dysplasia when starting treatment with CPAP compared with intubation and ventilation in preterm infants less than 30 weeks of gestation with respiratory distress. The meta-analysis reported no differences in the individual outcomes of mortality, bronchopulmonary dysplasia, pneumothorax, intraventricular hemorrhage, necrotizing enterocolitis, or retinopathy of prematurity.68
- One randomized trial enrolling 124 preterm infants (28 to 36 weeks of gestation) compared CPAP to high-flow nasal cannula as primary respiratory support in the delivery room.69 There was no difference in “treatment failure” within 24 hours between groups. Treatment failure was defined as oxygen concentration greater than 40%, failure of immediate extubation following surfactant administration by the Intubation-Surfactant-Extubation technique, predefined levels of acidosis or hypercarbia, apnea with predefined criteria of severity, or subsequent mechanical ventilation.
- Two RCTs,70,71 2 observational studies,72,73 and 1 meta-analysis74 inform the use of CPAP in the delivery room. The studies included both prophylactic use of CPAP for prevention of and treatment of respiratory distress. The use of CPAP for spontaneously breathing term and late preterm infants ≥34 weeks’ gestational age in the delivery room may decrease neonatal intensive care unit admission or respiratory support in the neonatal intensive care unit, though the incidence of pneumothorax may be increased.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. Oxygen supplementation should be titrated to target oxygen saturation goals in newborn infants receiving respiratory support. |
| 1 | C-LD | 2. A pulse oximeter should be placed as soon as possible for newborn infants receiving respiratory support or supplemental oxygen. |
| 2b | C-LD | 3. In term and late preterm newborn infants born at 35 weeks’ or more gestational age receiving respiratory support at birth, it may be reasonable to begin with 21% oxygen. |
| 2b | C-LD | 4. In preterm newborn infants born at 32 to 34+6/7 weeks’ gestational age receiving respiratory support at birth, it may be reasonable to begin with 21% to 30% oxygen. |
| 2b | C-LD | 5. In preterm newborn infants born at less than 32 weeks’ gestational age receiving respiratory support at birth, it may be reasonable to begin with 30% to 100% oxygen. |
Synopsis
At birth, the newborn infant’s blood oxygen levels rise over several minutes. For newborn infants who require resuscitation, supplemental oxygen may prevent harm from hypoxia.1 However, hyperoxia may also be associated with harm.2 The balance of benefit and harm of oxygen supplementation varies by gestational age and according to the individual newborn infant’s physiology.
Pulse oximetry allows oxygen titration to prevent either hypoxia or hyperoxia. Placement of a pulse oximeter sensor and acquisition of a reliable signal takes time. Appropriate sensor placement is preductal, on the infant’s right hand or wrist, as this reflects the blood oxygen saturation of the myocardium and brain.
In the very preterm population, failing to reach an oxygen saturation of 80% by 5 minutes has been associated with death regardless of initial oxygen concentration.3,4 For infants less than 32 weeks’ gestational age, initial oxygen concentrations of 21% to 30% may be insufficient to achieve target oxygen saturations.5
Recommendation-Specific Supportive Text
- Multiple studies have measured oxygen saturation in newborn infants at birth, but there are limited data on different oxygen saturation targets or titration strategies during newborn resuscitation. Optimal oxygen saturation targets for newborn infants receiving resuscitation at birth remain unknown. In the absence of such evidence, currently recommended preductal oxygen saturation goals remain based on ranges measured in healthy term infants after vaginal birth at sea level with the goal of avoiding the risks associated with hypoxia and hyperoxia.
- Placement of a pulse oximeter early in resuscitation results in earlier reading of oxygen saturation for guidance of oxygen therapy during resuscitation. In an observational study of videos of 230 resuscitated infants, median time to obtain pulse oximetry was 238 seconds.6 In an observational study comparing asphyxiated infants to controls, the median time for oxygen saturation detection was longer (260 seconds) than in controls (100 seconds).7 In an observational study of 428 infants, time taken to achieve an oxygen saturation reading in preterm infants was longer than in term infants.8
- For term and late preterm infants 35 or more weeks of gestation requiring respiratory support at birth, initiating resuscitation with 21% oxygen (air) compared with 100% oxygen is reasonable because evidence from meta-analysis suggests that it may reduce short-term mortality.9 The ILCOR systematic review of initial oxygen concentration for term and late preterm infants included 5 randomized and 5 quasi-randomized trials.9 Most of the evidence came from studies with a high risk of bias and all summary estimates had serious imprecision. Meta-analysis of 7 randomized and quasi-randomized trials showed decreased short-term mortality when using 21% oxygen compared with 100% oxygen.9 No difference was found in the risk of HIE or moderate-to-severe neurodevelopmental impairment among survivors at 1 to 3 years of age.9 Although this systematic review included randomized and quasi-randomized controlled trials, the LOE has been downgraded to C-LD because of the high risk of bias in the studies included and the serious imprecision for all reported outcomes. No studies have compared initiating resuscitation with intermediate oxygen concentrations (22%–99% oxygen).
- Evidence from meta-analysis of 10 randomized trials enrolling newborn infants less than 35 weeks’ gestational age found no difference in short-term mortality when respiratory support was started with low compared with high oxygen.10 In the included studies, low oxygen was generally 21% to 30% and high oxygen was 60% to 100%. In addition, no differences were found in multiple secondary outcomes. Most evidence came from studies with unclear risk of bias and all summary estimates had serious imprecision. In the subgroup of trials that used oxygen saturation targeting as a co-intervention, all preterm infants in whom respiratory support was initiated with 21% oxygen (air) required supplemental oxygen to achieve the predetermined oxygen saturation target. The recommendation to initiate respiratory support with a lower oxygen concentration reflects a preference to avoid exposing preterm newborn infants to excess oxygen beyond that necessary to achieve the predetermined oxygen saturation target without evidence demonstrating a benefit for important outcomes.11
- Analyses of the currently available data comparing initial oxygen concentration for very preterm infants have yielded conflicting conclusions, including for the critical outcome of mortality. A study-level meta-analysis of 10 RCTs enrolling preterm infants, including sub-analysis of 7 trials reporting outcomes for newborn infants 28 weeks’ gestational age or less, showed no difference in short-term mortality when respiratory support was started with low compared with high oxygen.10 In these studies, low oxygen was generally 21% to 30% and high oxygen was 60% to 100%. No differences were found in long-term mortality, neurodevelopmental outcome, retinopathy of prematurity, bronchopulmonary dysplasia, necrotizing enterocolitis, or major cerebral hemorrhage.10 In a systematic review of 8 trials that used oxygen saturation targeting as a co-intervention, all preterm newborns in whom respiratory support was initiated with 21% oxygen required supplemental oxygen to achieve the predetermined oxygen saturation target.10 A recent individual patient data meta-analysis found that in preterm infants less than 32 weeks of gestation, high initial (90% - 100%) oxygen was associated with lower mortality when compared with low initial (21% - 30%) oxygen concentrations.5
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | 1. In newborn infants, chest compressions are recommended if the heart rate remains less than 60/min despite 30 seconds of ventilation that inflates the lungs, as evidenced by chest movement. |
| 1 | C-EO | 2. During chest compressions in newborn infants, ventilation with an endotracheal tube is recommended. |
| 2b | C-EO | 3. During chest compressions for newborn infants born at 34 weeks’ or more gestational age when placement of an endotracheal tube is not possible or is unsuccessful, laryngeal mask may be a reasonable alternative. |
| 2b | C-EO | 4. It may be reasonable to use 100% oxygen during chest compressions in newborn infants. Once compressions are no longer needed, titrate the oxygen to meet saturation targets. |
Synopsis
Most newborn infants who require resuscitation at birth respond to initial steps of resuscitation or to ventilation that inflates the lungs. Effective ventilation is indicated by a rise in heart rate. Ventilation must be optimized before starting chest compressions, including placement of an alternative airway, if possible (intubation or laryngeal mask), as chest compressions compete with delivery of effective ventilation.1
Oxygen is essential for organ function; however, excess inspired oxygen during resuscitation may be harmful.2 Although current guidelines recommend using 100% oxygen during chest compressions,3 no studies have confirmed a benefit of using 100% oxygen compared to any other concentration, including air (21%).4 When providing chest compressions, the inspired oxygen can be increased to 100% and titrated to meet saturation targets once return of spontaneous circulation (ROSC) is achieved.
Recommendation-Specific Supportive Text
- Initiation of chest compressions in newborn infants with a heart rate less than 60/min is based on expert opinion because there are no clinical or physiological human studies addressing this question. A review article regarding strategies to prevent progression of bradycardia and the role of chest compressions for persistent neonatal bradycardia in the delivery room included some potentially useful animal data.5 Fetal lambs (n=14) were asphyxiated until cardiac arrest. Heart rates were continuously monitored and coronary, carotid, and pulmonary flows recorded and compared during asphyxia at different levels of bradycardia. Peak systolic carotid flows were significantly lower for heart rates Peak systolic carotid flows were significantly lower for heart rates less than 60/min compared to baseline.5
- In a neonatal simulation study, once chest compressions were initiated, health care professionals (n=30) were unable to recognize changing lung compliance and thus made little effort to optimize ventilation.1 Based on expert opinion, intubation is suggested during chest compressions in order to assure efficacy of ventilation, which is a priority for neonatal resuscitation.
- A recent ILCOR scoping review examined the evidence for using a laryngeal mask during chest compressions in newborn infants.6 Two animal studies suggest that ventilation via a laryngeal mask during chest compressions provides similar rates and time to ROSC compared to ventilation through an endotracheal tube.7,8 Animal and manikin data suggest that laryngeal masks can deliver comparable ventilation to that provided through an endotracheal tube during chest compressions.7-9
- A recent scoping review4 identified that to date there are no human studies comparing air, 100% oxygen, or any other oxygen concentration during newborn infant chest compressions. Animal studies have not found any difference in rates of ROSC, mortality, markers of injury or oxidative stress, hemodynamics or oxygen delivery.4,10-12 None of the animal studies examined any longer-term outcomes. Based on expert opinion, 100% oxygen during chest compressions is suggested. Once ROSC is achieved, oxygen should be weaned to meet goal saturations, as hyperoxia following resuscitation is associated with morbidity.13-15
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD | 1. It may be reasonable to repeatedly deliver 3 compressions followed by a ventilation breath (3:1 ratio) when providing chest compressions to newborn infants. |
| 2b | C-LD | 2. It may be reasonable to use the 2 thumb–encircling hands technique compared to the 2-finger or other techniques when providing chest compressions to newborn infants. |
| 2b | C-LD | 3. It may be reasonable to compress to one third the anterior-posterior diameter of the chest when providing chest compressions to newborn infants. |
| 2b | C-LD | 4. It may be reasonable to compress over the lower one third of the sternum, taking care to be above the xiphoid process when providing chest compressions to newborn infants. |
| 2b | C-LD | 5. It may be reasonable to change the compressor role every 2 to 5 minutes when providing chest compressions to newborn infants, and to switch the compressor during the heart rate assessment period. |
Synopsis
The need for chest compressions is uncommon for newborn infants.16,17 When compressions are indicated, the preferred compression to ventilation ratio is 3:1 in synchrony. The 2 thumb–encircling hands compression technique may have benefit over the 2-finger technique with respect to blood pressure generation and clinician fatigue. When providing chest compressions with the 2 thumb–encircling technique, the hands encircle the chest while the thumbs depress the sternum.18 Performing chest compressions with this technique at the head of the bed facilitates placement of an umbilical venous catheter and has ergonomic advantages over compressing from the side of the infant.19
Optimal blood flow during chest compressions is achieved by compressing the chest to one third the anteroposterior diameter over the lower third of the sternum, with care to avoid the xiphoid process to avoid liver injury. Compressor fatigue may be minimized through routine role rotation among team members, preferably during heart rate assessments to limit interruptions in compressions.
Recommendation-Specific Supportive Text
- In animal studies, the use of compression-to-ventilation ratios different from 3:1 (eg, 2:1, 4:1, 5:1, 9:3, 15:2) is associated with similar times to ROSC and mortality rates.20-25 The use of chest compressions with asynchronous ventilations compared to a ratio of 3:1 has varied results with inconsistencies between studies with some reporting improvements in ROSC and survival, others showing no difference and some showing deteriorations in depth of compression and compressor fatigue.4 A new technique of providing continuous compressions during a sustained inflation has been studied in animals4,11,26,27 as well as in a small pilot trial in newborn infants.28 The majority of studies suggest potential benefit with faster time to ROSC but inconsistent survival results. A larger clinical trial found no differences but was underpowered.29 Providing continuous compressions during a sustained inflation remains an interesting area of research but is not ready for clinical application.
- Animal and manikin studies frequently demonstrate greater chest compression depth, improved carotid blood flow, lower fatigue, and higher proportion of correct hand placement with the 2 thumb–encircling hands technique compared with the 2-finger and othertechniques.4,30 In 2 newborn infants with indwelling catheters, the 2 thumb–encircling hands technique generated higher systolic and mean blood pressures compared with the 2-finger technique.31
- In a randomized animal trial, time to ROSC was similar with chest compression depths of 25%, 33%, and 40% of the anteroposterior chest diameter. A chest compression depth of only 12.5% did not achieve ROSC.32 The same group also reported on hemodynamic parameters, with the highest carotid blood flow and systemic mean blood pressure being achieved with 40% chest compression depth.32 A retrospective clinical study examined chest computed tomography scans of 54 neonates and concluded that the recommended one third anteroposterior diameter would be more effective than one fourth anteroposterior diameter compression and safer than one half anteroposterior diameter compressions.33 No neonatal survival outcomes or long-term outcomes of newborn infants who received varying depths of compression are available.
- One of the earliest reports of newborn infant chest compressions compared abdominal versus xiphoid versus middle sternum versus simultaneous chest/abdominal compression. Fresh cadavers of infants and young children (n=15) were used with subsequent autopsy examination. No rupture of the liver was produced when pressure was applied to the chest alone at mid-sternum. Superficial tears of the liver capsule were produced when pressure was applied to the xiphoid process and all patients with simultaneous chest/abdominal or abdominal compressions alone had liver rupture. Compression of the middle part of the sternum (above the xiphoid) produced effective circulatory pressures.34 Chest radiograph studies with markers at the sternal notch and xiphoid process prior to taking the film subsequently identified that for the majority of infants, the heart lies under the lower one third of the sternum.35-37 Additional studies found that the nipple line is not a good landmark for determining compression position.38,39 Review of infant chest computed tomography scans to evaluate the optimal chest compression site found that the left ventricle was located beneath the lower quarter of the sternum the majority of the time.40 Due to the safety concerns from the older cadaveric studies, it is important to make sure the compression is above the xiphoid on the lower one thirdof the sternum.33
- High-quality chest compressions encompass multiple factors including optimal compression to ventilation ratio, adequate rate, depth of compression and full recoil between compressions. If the compressor experiences fatigue, the quality of the compressions declines.41,42 Neonatal manikin studies demonstrate that compression quality can diminish by 2 to 5 minutes when a single clinician performs compressions.43-46
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. Insertion of an umbilical vein catheter is recommended for newborn infants requiring emergency vascular access. |
| 2a | C-LD | 2. Insertion of an intraosseous vascular access device can be useful for newborn infants requiring emergency vascular access if intravenous access is not successful or not feasible. |
Synopsis
Newborn infants who do not respond to ventilation and chest compressions require emergency vascular access to administer epinephrine or volume expanders. Although few newborn infants receive emergency medications,1,2 health care professionals must be prepared to obtain rapid vascular access because delayed administration of epinephrine is associated with a decreased likelihood of survival.3 In the newborn infant, the umbilical vein is readily accessible and provides direct access to the heart and ascending aorta,4 but many health care professionals without neonatology specialty training perceive umbilical vein catheterization to be difficult.5 The insertion of an IO vascular access device into the medullary cavity of a long bone is an alternative route and is commonly used in pre-hospital and emergency department settings.6 IO vascular access devices have been associated with serious complications including fractures, extravasation leading to compartment syndrome, and tissue necrosis.7
Recommendation-Specific Supportive Text
- Historically, umbilical vein catheterization has been the standard approach to emergency vascular access in the delivery room and has been recommended since the first international consensus guidelines for neonatal resuscitation.8 In a registry study of 93,656 neonates in the delivery room, of the 47 neonates who received epinephrine, 32% achieved ROSC with endotracheal epinephrine while 77% achieved ROSC with intravascular epinephrine (by umbilical venous catheter) after failing endotracheal epinephrine.1 In a single-center retrospective case series, 15/20 (75%) of newborn infants who received the first dose of epinephrine through an umbilical vein catheter achieved ROSC.9 In an animal model of neonatal asphyxia, epinephrine infused through a low-lying umbilical vein catheter achieved comparable peak plasma epinephrine concentrations and ROSC when compared with direct injection into the right atrium.10 There are no human studies directly comparing the safety and effectiveness of umbilical vein catheters or IO vascular access devices, or describing the first attempt success and complication rate for umbilical vein catheterization during neonatal resuscitation.
- A systematic review, including 1 case series and 12 individual case reports, described successful IO vascular access device insertion among 41 neonates during resuscitation in the delivery room or neonatal intensive care unit.7 In total, 5 infants had serious complications, including 2 cases of limb ischemia leading to subsequent amputation and 1 tibial fracture. Subsequently, 3 case series have described IO vascular access device insertion among 12 neonates (10 newborn infants) identified by a survey from a single center,11 161 neonates (113 in the first day of life) identified from a multicenter pediatric emergency registry,12 and 102 neonates (13 in the delivery room) identified from records of a pediatric emergency transfer service.13 Successful IO vascular device insertion on the first attempt in neonates ranged from 50% to 86% with any complication reported in 10% to 35%. In an asphyxiated lamb model of neonatal resuscitation, the peak serum epinephrine concentration and ROSC rates were similar between the umbilical vein catheter and IO vascular access device groups.14 In simulation studies, participants inserted an IO vascular access device more quickly than an umbilical vein catheter.15-17 However, in an anatomic cadaver study (n=16; median gestational age, 29 weeks [IQR, 27–38 weeks] ) using 2 different IO vascular access devices, computed tomography imaging showed the tip of the device within the medullary cavity after only 39.7% to 61.1% of insertions.18
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In newborn infants whose heart rate has not increased to 60/min or more after optimizing ventilation and chest compressions, it is recommended to administer intravascular epinephrine (0.01–0.03 mg/kg). |
| 2a | B-NR | 2. In newborn infants who do not have an adequate response to an endotracheal dose of epinephrine, it is reasonable to administer intravascular epinephrine as soon as access is obtained regardless of the dosing interval. |
| 2a | C-LD | 3. In newborn infants receiving epinephrine, it is reasonable to administer endotracheal epinephrine at a larger dose (0.05–0.1 mg/kg) while vascular access is being obtained. |
| 2b | C-LD | 4. In newborn infants receiving epinephrine, it may be reasonable to administer additional doses of epinephrine every 3 to 5 minutes, preferably intravascularly, if the heart rate remains less than 60/min. |
Synopsis
Medications are rarely needed in resuscitation of the newborn infant because low heart rate usually results from poor gas exchange or inadequate lung inflation after birth. Establishing ventilation is the most important step in the resuscitation of a bradycardic newborn infant. However, if heart rate remains less than 60/min after ventilating with 100% oxygen (preferably through an alternative airway, eg, endotracheal tube or laryngeal mask) and chest compressions, administration of epinephrine is indicated. Epinephrine is a cardiac and vascular stimulant that improves blood flow to the coronaries and causes vasoconstriction to the peripheral vessels. Providing epinephrine in a newborn infant who remains bradycardic despite adequate ventilation may improve the heart rate and strength of contractions.
Administration of intravascular epinephrine (through either a low-lying umbilical venous catheter or IO vascular access device) provides the most rapid and reliable medication delivery. The bioavailability of epinephrine using either method should be similar and the choice of route may depend on the location of birth, training of health care professionals, team experience in vascular access, and available supplies. The intravascular dose of epinephrine is 0.01 to 0.03 mg/kg. This can be followed by a 3 mL normal saline flush regardless of birth weight.1 While intravascular access is in progress, epinephrine may be given by the endotracheal route in a dose of 0.05 to 0.1 mg/kg, as a higher dose is likely needed to achieve a similar level to intravascular administration.2-7
Recommendation-Specific Supportive Text
- A term piglet model of asphyxia-induced cardiac arrest demonstrated that intravenous epinephrine improved rates of ROSC compared to compressions alone.8 Observational evidence in human infants does not demonstrate greater efficacy of endotracheal or intravenous epinephrine; however, most babies received at least 1 intravenous dose before ROSC.2,9,10 Three studies using a term perinatal lamb model of asphyxia-induced cardiac arrest found that central venous epinephrine was associated with shorter time to ROSC and higher rates of ROSC than endotracheal epinephrine.11-13 In a similar model, IO epinephrine was as effective as intravenous.14 Intravenous epinephrine followed by a normal saline flush improves medication delivery.1,15
- In both a single-center observational study and a multicenter registry study, most infants who received an endotracheal dose achieved ROSC after subsequent intravenous doses.9,10 Although the more rapid response to intravenous epinephrine warrants its immediate administration once umbilical access is obtained, repetitive endotracheal doses or higher intravenous doses may result in potentially harmful plasma levels that lead to associated hypertension and tachycardia.16-19
- One small observational human study demonstrated 0.01 to 0.03 mg/kg to be an inadequate endotracheal dose.2 Both a single-center observational study and a multicenter registry study showed that although some newborn infants can achieve ROSC in response to endotracheal epinephrine while vascular access is being obtained, the majority need subsequent intravascular doses to achieve ROSC.9,10 In a perinatal model of cardiac arrest, peak plasma epinephrine concentrations in animals were higher and were achieved sooner after central or low-lying umbilical venous administration compared with the endotracheal route, despite a lower intravascular dose (0.03 mg/kg intravascular versus 0.1 mg/kg endotracheal route).13 Given these data, up to 0.1 mg/kg may be needed for an endotracheal dose to achieve adequate plasma concentrations.
- In a single-center observational study and a multicenter observational registry study, many infants received multiple doses of epinephrine before ROSC. 9,10 One perinatal model of cardiac arrest documented peak plasma epinephrine concentrations at 1 minute after intravenous administration, but not until 5 minutes after endotracheal administration. 13
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-EO | 1. It may be reasonable to administer a volume expander to newborn infants with evidence of hypovolemia, based on history and physical examination, who remain bradycardic (heart rate less than 60/min) despite ventilation, chest compressions, and epinephrine. |
| 2b | C-EO | 2. It may be reasonable to use normal saline (0.9% sodium chloride) or blood at 10 to 20 mL/kg for volume expansion. |
Synopsis
Newborn infants who continue to have respiratory and circulatory compromise after assisted ventilation, chest compressions, and epinephrine require further assessment and interventions. Tension pneumothorax requiring thoracentesis or acute blood loss that necessitates volume infusion can be considered. Newborn infants in shock from blood loss may respond poorly to the initial resuscitative efforts of ventilation, chest compressions, or epinephrine. History and physical exam findings suggestive of blood loss include a pale appearance, weak pulses, and persistent bradycardia (heart rate less than 60/min). Blood may be lost from the placenta into the mother’s circulation, from the cord, or from the infant.
When blood loss is suspected in a newborn infant who responds poorly to resuscitation, administering a volume expander without delay may improve circulation and perfusion. Normal saline (0.9% sodium chloride) is the crystalloid fluid of choice. Uncrossmatched type O, Rh-negative blood (or crossmatched, if immediately available) is preferred when blood loss is substantial.1,2 The recommended route is intravascular via an umbilical line, with the IO route being an alternative (refer to the Intravascular Access section).
Recommendation-Specific Supportive Text
- The recommendation for volume resuscitation at delivery is based on the recognition that extensive blood loss can occur at the time of birth leading to cardiorespiratory compromise in the newborn infant. Conditions such as uterine rupture may be noted by the obstetric team, while conditions such as fetal-maternal hemorrhage may be unrecognized. A newborn infant with decreased intravascular volume due to blood loss may not respond to resuscitative measures fully until there is increased oxygen carrying capacity and adequate cardiac preload provided by volume resuscitation. There is no evidence from randomized trials to support the use of routine volume resuscitation at delivery. One large retrospective review found that 0.04% of newborns received volume resuscitation in the delivery room, confirming that it is an uncommon event.3
- There is insufficient clinical evidence to determine what type of volume expander (crystalloid or blood) is more beneficial during neonatal resuscitation. Extrapolation from studies in hypotensive newborns shortly after birth4-6 and studies in animals (piglets) support the use of crystalloid over albumin solutions2 and blood over crystalloid solutions.1 The amount of volume expansion is based on usual practice in neonatal intensive care, 10 to 20 mL/kg initially, and repeating if inadequate response. In an animal study of asphyxia in the context of blood loss, providing 1 to 3 al iquot s of 10 mL/kg was associated with ROSC in 33/44 animals .1 While no clinical trials have established an optimal infusion rate, a steady infusion over 5 to 10 minutes may be reasonable.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. Newborn infants born at greater than 36 weeks’ gestational age with moderate-to-severe HIE should be offered therapeutic hypothermia under clearly defined protocols. |
| 1 | B-NR | 2. Newborn infants who receive prolonged ventilation or advanced resuscitation (intubation, chest compressions, or epinephrine) should be closely monitored, including checking glucose levels early in the post-resuscitation period and continuing until the glucose levels are maintained in the normal range. |
| 1 | C-LD | 3. For newborn infants who are unintentionally hypothermic (temperature less than 36 °C) after resuscitation, rewarming is indicated. The optimal rate of rewarming is unknown. |
Synopsis
Therapeutic hypothermia, lowering the newborn infant’s core temperature to a target temperature of 33.5 °C to 34.5 °C within 6 hours of birth for a duration of 72 hours, has the potential to improve outcomes for selected infants with neurologic injury, but careful implementation and monitoring are necessary.1 Newborn infants who receive prolonged ventilation or advanced resuscitation (eg, intubation, chest compressions, or epinephrine) should be closely monitored after stabilization in a neonatal intensive care unit or a monitored triage area because these infants are at risk for further deterioration. Monitoring may identify and prevent subsequent complications, including neurologic, metabolic or respiratory issues that can impact both short- and long-term outcomes for newborn infants.
Recommendation-Specific Supportive Text
- Infants 36 weeks’ or greater gestational age who receive advanced resuscitation should be examined for evidence of HIE to determine if they meet criteria for therapeutic hypothermia. Therapeutic hypothermia should be provided under defined protocols similar to those used in published clinical trials and in facilities capable of multidisciplinary care and longitudinal follow-up.1 Therapeutic hypothermia for infants less than 36 weeks’ gestational age with HIE has not shown benefit and may be associated with harm.2
In a meta-analysis of 8 RCTs involving 1344 term and late preterm infants with moderate-to-severe encephalopathy and evidence of intrapartum asphyxia, therapeutic hypothermia resulted in a significant reduction in the combined outcome of mortality or major neurodevelopmental disability at 18 months of age (OR, 0.75; 95% CI, 0.68–0.83).3 - Newborn infants who required advanced resuscitation, such as prolonged ventilation, chest compressions, or epinephrine, are at significant risk of developing moderate-to-severe HIE4-6 and other morbidities.7-9 Close monitoring to assess the need for interventions is warranted. This may involve care in a nursery with additional staffing, monitors, and equipment, or in a neonatal intensive care unit.
Hypoglycemia is common in infants who have received advanced resuscitation and is associated with poor outcomes.10,11 These infants should be monitored for hypoglycemia and treated appropriately. Newborn infants with abnormal glucose levels (both low and high) are at increased risk for brain injury and adverse outcomes after a hypoxic-ischemic insult.12-14 Retrospective chart review has shown risk of hypoglycemia after advanced resuscitation, especially in preterm infants born less than 32 weeks’ gestational age.11 - Infants with unintentional hypothermia (3. Infants with unintentional hypothermia (temperature less than 36 °C) immediately after stabilization should be rewarmed to avoid complications associated with low body temperature (including increased mortality, brain injury, hypoglycemia, sepsis, and respiratory distress). Evidence suggests that warming can be done rapidly (0.5 °C/h) or slowly (less than 0.5 °C/h) with no significant difference in outcomes.15-19 Three small RCTs15,16,20 and 6 observational studies17-19,21-23 of infants with hypothermia after delivery room stabilization found no difference between rapid or slow rewarming for outcomes of mortality,17-23 convulsions/seizures,16 intraventricular or pulmonary hemorrhage,16-18,20,21 hypoglycemia,15,16,18,20 or apnea.15,16,18,20 One observational study found less respiratory distress in infants who were slowly rewarmed,19 while a separate study found less respiratory distress syndrome in infants who were rapidly rewarmed.18 An RCT found no difference in respiratory distress or need for intubation.20
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In newborn infants receiving resuscitation, if there is no heart rate and all the steps of resuscitation have been performed, cessation of resuscitation efforts should be discussed with the team and the family. A reasonable time frame to consider this change in goals of care is around 20 minutes after birth |
| 1 | C-EO | 2. In newborn infants, noninitiation of resuscitation and discontinuation of life-sustaining treatment during or after resuscitation should be considered ethically equivalent |
| 2a | C-EO | 3. If a birth involves a condition likely to result in early death or severe morbidity of a newborn infant, noninitiation or limitation of resuscitation is reasonable after appropriate consultation and shared decision-making with the family. |
Synopsis
Experts agree that, in certain clinical conditions, it is reasonable to not initiate or discontinue life-sustaining efforts while continuing to provide supportive care for newborn infants and their families.1-7 If the heart rate remains undetectable and all steps of resuscitation have been completed by around 20 minutes after birth, it may be reasonable to redirect goals of care. Case series show few intact survivors after 20 minutes of no detectable heart rate.4 Variables to consider include whether resuscitation was considered optimal, availability of advanced neonatal care, circumstances before delivery, and the family’s wishes.4,7
Some newborn infants are so sick or immature at birth that survival is unlikely, even if resuscitation and intensive care are provided. In addition, some conditions are so severe that the burden of the illness and treatment greatly outweighs the likelihood of survival or a health outcome that aligns with the family’s wishes. These situations benefit from appropriate consultation and shared decision-making with families. Some of these scenarios may result in noninitiation or limitation of resuscitative efforts at birth, focusing on humane, compassionate, and culturally sensitive palliative care to ensure the newborn infant’s comfort.1,3-7
Recommendation-Specific Supportive Text
- 1.An ILCOR review of RCTs and observational studies in settings where therapeutic hypothermia is available describes variable rates of survival without moderate to severe disability in newborn infants who achieve ROSC after 10 minutes or more despite continued resuscitation. None of these studies evaluate outcomes of resuscitations that extend beyond 20 minutes of age, by which time the likelihood of intact survival was very low. The studies were too heterogeneous to be amenable to meta-analysis.4 More recent data from the Optimizing Cooling trial also demonstrated that an Apgar score of 0 at 10 minutes, in isolation, did not accurately predict death or significant neurodevelopmental impairment.8
- National medical societies agree that conditions exist for which it is reasonable to not initiate resuscitation, to limit resuscitation, or to discontinue resuscitation once these conditions are identified.1-6,9 Further aspects of medical ethics are addressed in “Part 3: Ethics.”10
- Conditions in which noninitiation, limitation, or discontinuation of resuscitation may be considered include extremely preterm birth and certain severe congenital anomalies. National guidelines recommend individualization of parent-informed decisions based on social, maternal, and fetal/neonatal factors.1-3,5,6 A systematic review showed that international guidelines variably described periviability between 22 weeks’ and 24 weeks’ gestational age.11
Training and Human Performance
There are 7 topics regarding education and training methods for resuscitation that are discussed in “Part 12: Resuscitation Education Science”1 and are relevant to neonatal resuscitation. The recommendations for these topics are summarized as follows:
- Spaced training (training or retraining distributed over time) may be considered rather than massed training (training provided at a single time point).
- Blended training (a combination of face-to-face and online instruction) is recommended compared to a nonblended training approach (either face-to-face or online instruction only).
- Rapid-cycle deliberate practice may be considered in instructional design.
- Gamified learning (use of game-like elements in the context of training, such as point systems, group competition, leaderboards, increasing levels of challenge, etc) may be considered as a component of resuscitation training.
- The teaching of teamwork competencies may be considered during resuscitation training.
- High-fidelity manikin use may be considered during resuscitation training when local resources allow. In the absence of such resources, low-fidelity manikins may be used.
- Augmented reality (hands-on experience with addition of virtual content) or traditional training may be considered for resuscitation training. The use of virtual reality alone is not recommended.
Please refer to “Part 12: Resuscitation Education Science” for the full recommendations and additional information on these topics.1
Knowledge Gaps and Priorities for Research
Neonatal resuscitation science continues to advance, with contributions by many researchers in laboratories, in the delivery room, and in other clinical settings. This research has led to refinement and improvements in the Neonatal Resuscitation Algorithm. It has also led to the recognition of gaps in knowledge to further optimize neonatal resuscitation. Innovative study designs such as cluster randomization and stepped wedge designs, along with changes in consent processes informed by families may further advance neonatal resuscitation research. Review of the knowledge chunks during this update identified questions and practices for which evidence level was low, uncertain, or absent. The following knowledge gaps would benefit from further research:
Resuscitation Preparedness
- Specific maternal and fetal factors that may warrant the need for an advanced resuscitation team
- Current rates of various interventions during neonatal resuscitation in different birth settings
During and Just After Birth
- Optimal cord management strategies for specific populations, including multiple gestation, moderately preterm infants, and those with congenital heart or lung disease
- Optimal duration of deferred cord clamping for preterm gestational age groups
- Specific clinical indications for immediate or early cord clamping
Early Resuscitation
- Adjunctive interventions that may optimize outcomes during deferred cord clamping
- Optimal interface for initial ventilation for term and preterm newborn infants
- Effectiveness of laryngeal mask for newborn infants less than 34 weeks’ gestational age or less than 2 kg birth weight
- Optimal ventilation rate and whether it may depend on gestational age
- Effectiveness of new technologies for rapid heart rate measurement in regard to accuracy and clinical outcomes
- Optimal oxygen management during and after resuscitation, including titration strategies
Advanced Resuscitation
- Optimal techniques for chest compressions in newborn infants including ratio of compressions to ventilations, asynchronous versus synchronous technique, and method of compression
- Optimal airway management during chest compressions, such as the use of a laryngeal mask and its effectiveness compared to ventilation delivered by endotracheal tube
- Whether certain populations outside of the immediate birth period may have better outcomes when resuscitated by using the neonatal or the pediatric resuscitation algorithm
- Optimal mode of intravascular access outside of the delivery room setting to administer medications or fluids
- Optimal timing, dosing, dose interval, and delivery routes for epinephrine, including earlier use in newborn infants requiring extensive advanced resuscitation
- Indications for volume expansion, as well as optimal dosing, timing, and type of volume
- The recognition and management of pulseless electrical activity
- Passive cooling during resuscitation in infants with moderate to severe HIE
Postresuscitation Care
- Optimal dose, route, and timing of surfactant in at-risk newborn infants, including less-invasive administration techniques
- Indications for and effectiveness of CPAP for various populations
- Strategies to prevent subsequent hypothermia after the immediate birth period
- Optimal rewarming strategy for newborn infants with unintentional hypothermia
- Strategy for blood glucose monitoring after neonatal resuscitation, including appropriate patient selection, method, and frequency of measurement
- Effectiveness of therapeutic hypothermia for mild HIE
Education and Maintenance of Skills
- Optimal training strategies for individual and team performance, including methods of learning, frequency, and adjunctive tools such as virtual reality
For these gaps, when relevant and feasible, it is important to select outcomes considered critical or important by both health care professionals and families of newborn infants. The research community should seek to design studies that will lead to evidence with a high level of certainty. Internal validity is ideally addressed by clearly defined primary outcomes, appropriate sample sizes, relevant and timed interventions and controls, and time series analyses in implementation studies. ILCOR has published a guideline that should be followed for uniform reporting of neonatal resuscitation research.1 External validity can be optimized by studying relevant health care professional populations and systems of care, and by measuring the impact on critical patient outcomes.
Researchers studying these gaps will need to consider innovations in clinical trial design; examples include pragmatic study designs and novel consent processes. As mortality and severe morbidities decline with advancements and improvements in health care delivery, having adequate power for some clinical questions using traditional individual patient randomized trials declines. Another barrier is the difficulty in obtaining antenatal consent for clinical trials in the delivery room. Adaptive trials and those using cluster randomization may be suitable for some questions. When feasible, well-designed multicenter randomized clinical trials should be used to generate the highest-quality evidence.
Research should address the values and preferences of our key stakeholders, the families and teams who are involved in the process of resuscitation. Research in neonatal resuscitation has been noted to have underrepresentation of various populations. In the context of disparities in care and outcomes researchers can make efforts to elicit input from representative families and work to ensure appropriate recruitment in trials.
The American Heart Association requests that this document be cited as follows: Lee HC, Strand ML, Finan E, Illuzzi J, Kamath-Rayne BD, Kapadia V, Mahgoub M, Niermeyer S, Schexnayder SM, Schmolzer GM, Weglarz J, Williams AL, Weiner GM, Wyckoff M, Yamada NK, Szyld E. Part 5: neonatal resuscitation: 2025 American Heart Association and American Academy of Pediatrics Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2025;152(suppl 2):S385–S423. doi: 10.1161/CIR.0000000000001367
This article has been copublished in Pediatrics.
- Henry C. Lee, MD, Co-Chair
- Marya L. Strand, MD, MS
- Emer Finan, MD
- Jessica Illuzzi, MD
- Beena D. Kamath-Rayne, MD, MPH
- Vishal Kapadia, MD, MSCS
- Melissa Mahgoub, PhD
- Susan Niermeyer, MD, MPH
- Stephen M. Schexnayder, MD
- Georg M. Schmölzer, MD
- Jessica Weglarz, MBA
- Amanda L. Williams, RN
- Gary M. Weiner, MD
- Myra Wyckoff, MD
- Nicole K. Yamada, MD, MS
- Edgardo Szyld, MD, MSc, Co-Chair
Open table in a new window.
Open table in a new window.
 Login
Login